Cancer testis antigen subfamilies: Attractive targets for therapeutic vaccine (Review)
- PMID: 37144487
- PMCID: PMC10198712
- DOI: 10.3892/ijo.2023.5519
Cancer testis antigen subfamilies: Attractive targets for therapeutic vaccine (Review)
Abstract
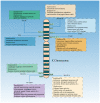
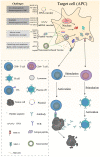
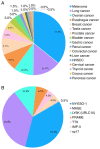
Cancer‑testis antigen (CTA) is a well‑accepted optimal target library for cancer diagnosis and treatment. Most CTAs are located on the X chromosome and aggregate into large gene families, such as the melanoma antigen, synovial sarcoma X and G antigen families. Members of the CTA subfamily are usually co‑expressed in tumor tissues and share similar structural characteristics and biological functions. As cancer vaccines are recommended to induce specific antitumor responses, CTAs, particularly CTA subfamilies, are widely used in the design of cancer vaccines. To date, DNA, mRNA and peptide vaccines have been commonly used to generate tumor‑specific CTAs in vivo and induce anticancer effects. Despite promising results in preclinical studies, the antitumor efficacy of CTA‑based vaccines is limited in clinical trials, which may be partially attributed to weak immunogenicity, low efficacy of antigen delivery and presentation processes, as well as a suppressive immune microenvironment. Recently, the development of nanomaterials has enhanced the cancer vaccination cascade, improved the antitumor performance and reduced off‑target effects. The present study provided an in‑depth review of the structural characteristics and biofunctions of the CTA subfamilies, summarised the design and utilisation of CTA‑based vaccine platforms and provided recommendations for developing nanomaterial‑derived CTA‑targeted vaccines.
Keywords: cancer vaccine; cancer‑testis antigen; gene family; melanoma antigen; nanomaterial delivery system; synovial sarcoma X.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers.Immunol Invest. 2016 Oct;45(7):619-40. doi: 10.1080/08820139.2016.1197241. Epub 2016 Sep 7. Immunol Invest. 2016. PMID: 27603913 Review.
-
Oncogenic cancer/testis antigens are a hallmarker of cancer and a sensible target for cancer immunotherapy.Biochim Biophys Acta Rev Cancer. 2021 Aug;1876(1):188558. doi: 10.1016/j.bbcan.2021.188558. Epub 2021 Apr 29. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33933558 Review.
-
Cancer/testis antigens: from serology to mRNA cancer vaccine.Semin Cancer Biol. 2021 Nov;76:218-231. doi: 10.1016/j.semcancer.2021.04.016. Epub 2021 Apr 25. Semin Cancer Biol. 2021. PMID: 33910064 Review.
-
The potential immune-eliciting cancer testis antigens in colorectal cancer.Immunotherapy. 2018 Sep;10(12):1093-1104. doi: 10.2217/imt-2018-0044. Immunotherapy. 2018. PMID: 30185136 Review.
-
Cancer-testis antigens: Unique cancer stem cell biomarkers and targets for cancer therapy.Semin Cancer Biol. 2018 Dec;53:75-89. doi: 10.1016/j.semcancer.2018.08.006. Epub 2018 Aug 29. Semin Cancer Biol. 2018. PMID: 30171980 Review.
Cited by
-
SPEF1 and SPEF2 as potential biomarkers in bladder cancer: Insights from a comprehensive bioinformatic analysis.Bladder (San Franc). 2025 Apr 11;12(2):e21200039. doi: 10.14440/bladder.2024.0071. eCollection 2025. Bladder (San Franc). 2025. PMID: 40747466 Free PMC article.
-
An Engineered M13 Filamentous Nanoparticle as an Antigen Carrier for a Malignant Melanoma Immunotherapeutic Strategy.Viruses. 2024 Feb 1;16(2):232. doi: 10.3390/v16020232. Viruses. 2024. PMID: 38400008 Free PMC article.
-
Identification of new HLA-A*0201-restricted cytotoxic T lymphocyte epitopes from LDHC in lung adenocarcinoma.Front Immunol. 2025 Apr 9;16:1564731. doi: 10.3389/fimmu.2025.1564731. eCollection 2025. Front Immunol. 2025. PMID: 40270965 Free PMC article.
-
Fusion of NY-ESO-1 epitope with heat shock protein 70 enhances its induced immune responses and antitumor activity against glioma in vitro.Transl Cancer Res. 2024 Jan 31;13(1):191-201. doi: 10.21037/tcr-23-1476. Epub 2024 Jan 19. Transl Cancer Res. 2024. PMID: 38410235 Free PMC article.
-
The human testis-specific protein Y-linked (TSPY) is a male-specific cancer-testis antigen capable of eliciting significant immune responses and elimination of positive tumor cells in hepatocellular carcinoma.Cell Biosci. 2025 Jun 25;15(1):88. doi: 10.1186/s13578-025-01432-8. Cell Biosci. 2025. PMID: 40563082 Free PMC article.
References
-
- Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–378. - PubMed
-
- Marchand M, van Baren N, Weynants P, Brichard V, Dréno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
