Non-Contact Irreversible Electroporation in the Esophagus With a Wet Electrode Approach
- PMID: 37144889
- PMCID: PMC10259469
- DOI: 10.1115/1.4062491
Non-Contact Irreversible Electroporation in the Esophagus With a Wet Electrode Approach
Abstract
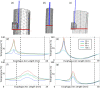
Our objective was to develop a technique for performing irreversible electroporation (IRE) of esophageal tumors while mitigating thermal damage to the healthy lumen wall. We investigated noncontact IRE using a wet electrode approach for tumor ablation in a human esophagus with finite element models for electric field distribution, joule heating, thermal flux, and metabolic heat generation. Simulation results indicated the feasibility of tumor ablation in the esophagus using an catheter mounted electrode immersed in diluted saline. The ablation size was clinically relevant, with substantially lesser thermal damage to the healthy esophageal wall when compared to IRE performed by placing a monopolar electrode directly into the tumor. Additional simulations were used to estimate ablation size and penetration during noncontact wet-electrode IRE (wIRE) in the healthy swine esophagus. A novel catheter electrode was manufactured and wIRE evaluated in seven pigs. wIRE was performed by securing the device in the esophagus and using diluted saline to isolate the electrode from the esophageal wall while providing electric contact. Computed tomography and fluoroscopy were performed post-treatment to document acute lumen patency. Animals were sacrificed within four hours following treatment for histologic analysis of the treated esophagus. The procedure was safely completed in all animals; post-treatment imaging revealed intact esophageal lumen. The ablations were visually distinct on gross pathology, demonstrating full thickness, circumferential regions of cell death (3.52 ± 0.89 mm depth). Acute histologic changes were not evident in nerves or extracellular matrix architecture within the treatment site. Catheter directed noncontact IRE is feasible for performing penetrative ablations in the esophagus while avoiding thermal damage.
Keywords: Arrhenius thermal damage; animal experiments; bioheat; finite element model; in vivo validation; irreversible electroporation; joule heating; medical device design; tissue ablation.
Copyright © 2023 by ASME.
Figures









References
-
- Haefner, M. F. , Lang, K. , Krug, D. , Koerber, S. A. , Uhlmann, L. , Kieser, M. , Debus, J. , and Sterzing, F. , 2015, “ Prognostic Factors, Patterns of Recurrence and Toxicity for Patients With Esophageal Cancer Undergoing Definitive Radiotherapy or Chemo-Radiotherapy,” J. Radiat. Res, 56(4), pp. 742–749. 10.1093/jrr/rrv022 - DOI - PMC - PubMed
-
- Dallal, H. J. , Smith, G. D. , Grieve, D. C. , Ghosh, S. , Penman, I. D. , and Palmer, K. R. , 2001, “ A Randomized Trial of Thermal Ablative Therapy Versus Expandable Metal Stents in the Palliative Treatment of Patients With Esophageal Carcinoma,” Gastrointest. Endosc., 54(5), pp. 549–557. 10.1067/mge.2001.118947 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

