Skeletal Editing Approach to Bridge-Functionalized Bicyclo[1.1.1]pentanes from Azabicyclo[2.1.1]hexanes
- PMID: 37145091
- PMCID: PMC10281541
- DOI: 10.1021/jacs.3c02616
Skeletal Editing Approach to Bridge-Functionalized Bicyclo[1.1.1]pentanes from Azabicyclo[2.1.1]hexanes
Abstract
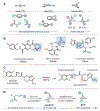
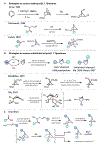
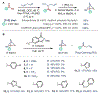
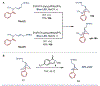
Azabicyclo[2.1.1]hexanes (aza-BCHs) and bicyclo[1.1.1]pentanes (BCPs) have emerged as attractive classes of sp3-rich cores for replacing flat, aromatic groups with metabolically resistant, three-dimensional frameworks in drug scaffolds. Strategies to directly convert, or "scaffold hop", between these bioisosteric subclasses through single-atom skeletal editing would enable efficient interpolation within this valuable chemical space. Herein, we describe a strategy to "scaffold hop" between aza-BCH and BCP cores through a nitrogen-deleting skeletal edit. Photochemical [2+2] cycloadditions, used to prepare multifunctionalized aza-BCH frameworks, are coupled with a subsequent deamination step to afford bridge-functionalized BCPs, for which few synthetic solutions currently exist. The modular sequence provides access to various privileged bridged bicycles of pharmaceutical relevance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
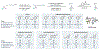

References
-
- Campos KR; Coleman PJ; Alvarez JC; Dreher SD; Garbaccio RM; Terrett NK; Tillyer RD; Truppo MD; Parmee ER The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363 (6424), No. eaat0805. - PubMed
-
- Mykhailiuk PK Saturated Bioisosteres of Benzene: Where to Go Next? Org. Biomol. Chem 2019, 17 (11), 2839–2849. - PubMed
-
- Subbaiah MAM; Meanwell NA Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem 2021, 64 (19), 14046–14128. - PubMed
-
- Stepan AF; Subramanyam C; Efremov IV; Dutra JK; O’Sullivan TJ; DiRico KJ; McDonald WS; Won A; Dorff PH; Nolan CE; Becker SL; Pustilnik LR; Riddell DR; Kauffman GW; Kormos BL; Zhang L; Lu Y; Capetta SH; Green ME; Karki K; Sibley E; Atchison KP; Hallgren AJ; Oborski CE; Robshaw AE; Sneed B; O’Donnell CJ Application of the Bicyclo[1.1.1]Pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem 2012, 55 (7), 3414–3424. - PubMed

