Hypoxia-inducible factor orchestrates adenosine metabolism to promote liver cancer development
- PMID: 37146141
- PMCID: PMC10162666
- DOI: 10.1126/sciadv.ade5111
Hypoxia-inducible factor orchestrates adenosine metabolism to promote liver cancer development
Abstract
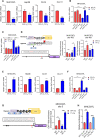
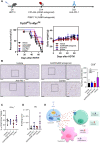
Hypoxia-induced adenosine creates an immunosuppressive tumor microenvironment (TME) and dampens the efficacy of immune checkpoint inhibitors (ICIs). We found that hypoxia-inducible factor 1 (HIF-1) orchestrates adenosine efflux through two steps in hepatocellular carcinoma (HCC). First, HIF-1 activates transcriptional repressor MXI1, which inhibits adenosine kinase (ADK), resulting in the failure of adenosine phosphorylation to adenosine monophosphate. This leads to adenosine accumulation in hypoxic cancer cells. Second, HIF-1 transcriptionally activates equilibrative nucleoside transporter 4, pumping adenosine into the interstitial space of HCC, elevating extracellular adenosine levels. Multiple in vitro assays demonstrated the immunosuppressive role of adenosine on T cells and myeloid cells. Knockout of ADK in vivo skewed intratumoral immune cells to protumorigenic and promoted tumor progression. Therapeutically, combination treatment of adenosine receptor antagonists and anti-PD-1 prolonged survival of HCC-bearing mice. We illustrated the dual role of hypoxia in establishing an adenosine-mediated immunosuppressive TME and offered a potential therapeutic approach that synergizes with ICIs in HCC.
Figures





References
-
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
-
- J. M. Llovet, S. Ricci, V. Mazzaferro, P. Hilgard, E. Gane, J.-F. Blanc, A. C. De Oliveira, A. Santoro, J.-L. Raoul, A. Forner, M. Schwartz, C. Porta, S. Zeuzem, L. Bolondi, T. F. Greten, P. R. Galle, J.-F. Seitz, I. Borbath, D. Häussinger, T. Giannaris, M. Shan, M. Moscovici, D. Voliotis, J. Bruix; SHARP Investigators Study Group , Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008). - PubMed
-
- M. Kudo, R. S. Finn, S. Qin, K.-H. Han, K. Ikeda, F. Piscaglia, A. Baron, J.-W. Park, G. Han, J. Jassem, J. F. Blanc, A. Vogel, D. Komov, T. R. J. Evans, C. Lopez, C. Dutcus, M. Guo, K. Saito, S. Kraljevic, T. Tamai, M. Ren, A.-L. Cheng, Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018). - PubMed
-
- A. B. El-Khoueiry, B. Sangro, T. Yau, T. S. Crocenzi, M. Kudo, C. Hsu, T.-Y. Kim, S.-P. Choo, J. Trojan, T. H. Welling III, T. Meyer, Y.-K. Kang, W. Yeo, A. Chopra, J. Anderson, C. D. Cruz, L. Lang, J. Neely, H. Tang, H. B. Dastani, I. Melero, Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017). - PMC - PubMed
-
- R. S. Finn, S. Qin, M. Ikeda, P. R. Galle, M. Ducreux, T.-Y. Kim, M. Kudo, V. Breder, P. Merle, A. O. Kaseb, D. Li, W. Verret, D.-Z. Xu, S. Hernandez, J. Liu, C. Huang, S. Mulla, Y. Wang, H. Y. Lim, A. X. Zhu, A.-L. Cheng; IMbrave150 Investigators , Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

