First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis
- PMID: 37146227
- PMCID: PMC10158799
- DOI: 10.1002/14651858.CD013798.pub2
First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis
Abstract
Background: Since the approval of tyrosine kinase inhibitors, angiogenesis inhibitors and immune checkpoint inhibitors, the treatment landscape for advanced renal cell carcinoma (RCC) has changed fundamentally. Today, combined therapies from different drug categories have a firm place in a complex first-line therapy. Due to the large number of drugs available, it is necessary to identify the most effective therapies, whilst considering their side effects and impact on quality of life (QoL).
Objectives: To evaluate and compare the benefits and harms of first-line therapies for adults with advanced RCC, and to produce a clinically relevant ranking of therapies. Secondary objectives were to maintain the currency of the evidence by conducting continuous update searches, using a living systematic review approach, and to incorporate data from clinical study reports (CSRs).
Search methods: We searched CENTRAL, MEDLINE, Embase, conference proceedings and relevant trial registries up until 9 February 2022. We searched several data platforms to identify CSRs.
Selection criteria: We included randomised controlled trials (RCTs) evaluating at least one targeted therapy or immunotherapy for first-line treatment of adults with advanced RCC. We excluded trials evaluating only interleukin-2 versus interferon-alpha as well as trials with an adjuvant treatment setting. We also excluded trials with adults who received prior systemic anticancer therapy if more than 10% of participants were previously treated, or if data for untreated participants were not separately extractable.
Data collection and analysis: All necessary review steps (i.e. screening and study selection, data extraction, risk of bias and certainty assessments) were conducted independently by at least two review authors. Our outcomes were overall survival (OS), QoL, serious adverse events (SAEs), progression-free survival (PFS), adverse events (AEs), the number of participants who discontinued study treatment due to an AE, and the time to initiation of first subsequent therapy. Where possible, analyses were conducted for the different risk groups (favourable, intermediate, poor) according to the International Metastatic Renal-Cell Carcinoma Database Consortium Score (IMDC) or the Memorial Sloan Kettering Cancer Center (MSKCC) criteria. Our main comparator was sunitinib (SUN). A hazard ratio (HR) or risk ratio (RR) lower than 1.0 is in favour of the experimental arm.
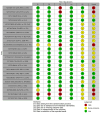
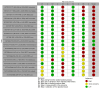
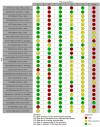
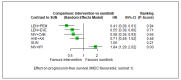
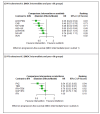
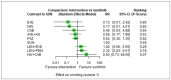
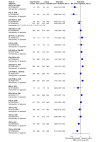
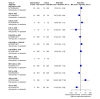
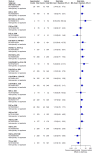
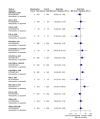
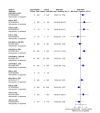
Main results: We included 36 RCTs and 15,177 participants (11,061 males and 4116 females). Risk of bias was predominantly judged as being 'high' or 'some concerns' across most trials and outcomes. This was mainly due to a lack of information about the randomisation process, the blinding of outcome assessors, and methods for outcome measurements and analyses. Additionally, study protocols and statistical analysis plans were rarely available. Here we present the results for our primary outcomes OS, QoL, and SAEs, and for all risk groups combined for contemporary treatments: pembrolizumab + axitinib (PEM+AXI), avelumab + axitinib (AVE+AXI), nivolumab + cabozantinib (NIV+CAB), lenvatinib + pembrolizumab (LEN+PEM), nivolumab + ipilimumab (NIV+IPI), CAB, and pazopanib (PAZ). Results per risk group and results for our secondary outcomes are reported in the summary of findings tables and in the full text of this review. The evidence on other treatments and comparisons can also be found in the full text. Overall survival (OS) Across risk groups, PEM+AXI (HR 0.73, 95% confidence interval (CI) 0.50 to 1.07, moderate certainty) and NIV+IPI (HR 0.69, 95% CI 0.69 to 1.00, moderate certainty) probably improve OS, compared to SUN, respectively. LEN+PEM may improve OS (HR 0.66, 95% CI 0.42 to 1.03, low certainty), compared to SUN. There is probably little or no difference in OS between PAZ and SUN (HR 0.91, 95% CI 0.64 to 1.32, moderate certainty), and we are uncertain whether CAB improves OS when compared to SUN (HR 0.84, 95% CI 0.43 to 1.64, very low certainty). The median survival is 28 months when treated with SUN. Survival may improve to 43 months with LEN+PEM, and probably improves to: 41 months with NIV+IPI, 39 months with PEM+AXI, and 31 months with PAZ. We are uncertain whether survival improves to 34 months with CAB. Comparison data were not available for AVE+AXI and NIV+CAB. Quality of life (QoL) One RCT measured QoL using FACIT-F (score range 0 to 52; higher scores mean better QoL) and reported that the mean post-score was 9.00 points higher (9.86 lower to 27.86 higher, very low certainty) with PAZ than with SUN. Comparison data were not available for PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, and CAB. Serious adverse events (SAEs) Across risk groups, PEM+AXI probably increases slightly the risk for SAEs (RR 1.29, 95% CI 0.90 to 1.85, moderate certainty) compared to SUN. LEN+PEM (RR 1.52, 95% CI 1.06 to 2.19, moderate certainty) and NIV+IPI (RR 1.40, 95% CI 1.00 to 1.97, moderate certainty) probably increase the risk for SAEs, compared to SUN, respectively. There is probably little or no difference in the risk for SAEs between PAZ and SUN (RR 0.99, 95% CI 0.75 to 1.31, moderate certainty). We are uncertain whether CAB reduces or increases the risk for SAEs (RR 0.92, 95% CI 0.60 to 1.43, very low certainty) when compared to SUN. People have a mean risk of 40% for experiencing SAEs when treated with SUN. The risk increases probably to: 61% with LEN+PEM, 57% with NIV+IPI, and 52% with PEM+AXI. It probably remains at 40% with PAZ. We are uncertain whether the risk reduces to 37% with CAB. Comparison data were not available for AVE+AXI and NIV+CAB.
Authors' conclusions: Findings concerning the main treatments of interest comes from direct evidence of one trial only, thus results should be interpreted with caution. More trials are needed where these interventions and combinations are compared head-to-head, rather than just to SUN. Moreover, assessing the effect of immunotherapies and targeted therapies on different subgroups is essential and studies should focus on assessing and reporting relevant subgroup data. The evidence in this review mostly applies to advanced clear cell RCC.
Trial registration: ClinicalTrials.gov NCT00065468 NCT00072046 NCT00081614 NCT00098657/NCT00083889 NCT00117637 NCT00126594 NCT00334282 NCT00420888 NCT00609401 NCT00619268 NCT00631371 NCT00719264 NCT00720941 NCT00732914 NCT00738530 NCT00903175 NCT00920816 NCT00979966 NCT01024920 NCT01030783 NCT01064310 NCT01108445 NCT01274273 NCT01392183 NCT01481870 NCT01613846 NCT01835158 NCT01984242 NCT02231749 NCT02420821 NCT02684006 NCT02761057 NCT02811861 NCT02853331 NCT03141177.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AAa: The grant by the German Federal Ministry of Education and Research does not lead to a conflict of interest. She is editor at Cochrane, but was not involved in the editorial process for this review.
BB: none known.
AAb: The grant by the German Federal Ministry of Education and Research does not lead to a conflict of interest. She is editor at Cochrane, but was not involved in the editorial process for this review.
IM: none known. She is information specialist for Cochrane Haematology, but was not involved in the editorial process for this review.
VP: none known.
ET: none known.
CH: none known.
ND: none known.
PM: none known.
PD: none known; he is Co‐ordinating Editor of Cochrane Urology, but was not involved in the editorial process for this review.
MG: none known.
AH: speaker honorary and research grant for renal cell carcinoma by BMS; however, this does not lead to a conflict of interest.
NS: none known; she is Co‐ordinating Editor of Cochrane Haematology, but was not involved in the editorial process for this review.
Figures



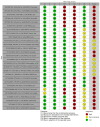

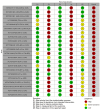
























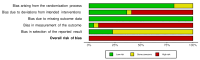

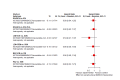



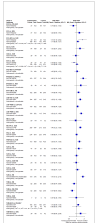

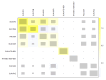




















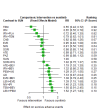





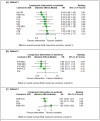



Update of
References
References to studies included in this review
Jonasch 2010 {published data only}
-
- Jonasch E, Corn P, Asche RG. Randomized phase II study of sorafenib with or without low-dose IFN in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2007;25(18 Suppl):Abstract 5104.
NCT00065468 {published data only}
-
- Atkins MB, Hidalgo M, Stadler W, Logan T, Dutcher JP, Hudes G, et al. A randomized double-blind phase 2 study of intravenous CCI-779 administered weekly to patients with advanced renal cell carcinoma. In: Proceedings of the American Society of Clinical Oncology. Vol. 21 (Pt 1). 2002:10a, Abstract 36. [ClinicalTrials.gov: NCT00065468]
-
- Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Annals of Oncology 2008;19(8):1387-92. [ClinicalTrials.gov: NCT00065468] - PubMed
-
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. A phase 3, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2006;24(18S):LBA4. [ClinicalTrials.gov: NCT00065468]
-
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New England Journal of Medicine 2007;356(22):2271-81. [ClinicalTrials.gov: NCT00065468] - PubMed
-
- Rajagopalan S, Pullenayegum E, Alemao E, Strahs A, Purvis J. Evaluation of adverse event-related hospitalizations in patients with advanced renal cell carcinoma on treatment with temsirolimus or interferon-alfa: results from a phase 3 randomized trial. European Journal of Cancer, Supplement 2009;7(2-3):441. [ClinicalTrials.gov: NCT00065468]
NCT00072046 {published data only}
-
- Halabi S, Rini BI, Stadler WM, Small EJ. Use of progression-free survival (PFS) to predict overall survival (OS) in patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2010;28(15 Suppl 1):4525-4525. [ClinicalTrials.gov: NCT00072046]
-
- Melichar B, Bracarda S, Bellmunt J, Ravaud A, Negrier S, Jethwa S, et al. First-line bevacizumab + reduced-dose interferon-alpha2a in patients (pts) with metastatic renal cell carcinoma (mRCC): An update on overall survival. European Journal of Cancer, Supplement 2009;7(2-3):430. [ClinicalTrials.gov: NCT00072046]
-
- Nixon AB, Halabi S, Liu Y, Starr MD, Brady JC, Shterev I, et al. Predictive biomarkers of overall survival in patients with metastatic renal cell carcinoma treated with IFNalpha +/- bevacizumab: results from CALGB 90206 (alliance). Clinical Cancer Research 2021;OF1 - OF8:29. [ClinicalTrials.gov: NCT00072046] - PMC - PubMed
-
- Rini BI, Halabi S, Rosenberg J, Stadler WM, Vaena DA, Atkins JN, et al. Bevacizumab plus interferon-alpha versus interferon-alpha monotherapy in patients with metastatic renal cell carcinoma: results of overall survival for CALGB 90206. Journal of Clinical Oncology 2009;27(15S Pt 1):239. [ClinicalTrials.gov: NCT00072046]
-
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of Clinical Oncology 2010;28(13):2137-43. [ClinicalTrials.gov: NCT00072046] - PMC - PubMed
NCT00081614 {published data only}
-
- Bukowski RM, Kabbinavar F, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, et al. Bevacizumab with or without erlotinib in metastatic renal cell carcinoma (RCC). Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2006;24(18S):4523. [ClinicalTrials.gov: NCT00081614]
-
- Bukowski RM, Kabbinavar FF, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. Journal of Clinical Oncology 2007;25(29):4536-41. [ClinicalTrials.gov: NCT00081614] - PubMed
NCT00098657/NCT00083889 {published data only}
-
- Blagoev KB, Wilkerson J, Stein WD, Motzer RJ, Bates SE, Fojo AT. Effect of sunitinib (Su) administration on post-treatment survival in patients with metastatic renal cell carcinoma (mRCC) treated on the upfront randomized phase III trial of sunitinib or interferon alfa (IFN). Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2011;29(15 Suppl 1):4634-4634. [ClinicalTrials.gov: NCT00098657/NCT00083889]
-
- Bushmakin A, Cappelleri JC, Korytowsky B, Sandin R, Matczak E, Cella D. Sunitinib (Su) dosing schedule and data collection timepoints: Impact on quality of life (QoL) outcomes in metastatic renal cell carcinoma (mRCC). Annals of Oncology 2012;9:ix269. [ClinicalTrials.gov: NCT00098657/NCT00083889]
-
- Cappelleri JC, Bushmakin AG, Charbonneau C, Kim ST, Li JZ, Motzer RJ. Quality of life (QoL) with sunitinib versus interferon-alfa (IFN) as first-line therapy in patients with metastatic renal cell carcinoma (mRCC): final results. Journal of Clinical Oncology 2009;27(15S Pt I):330. [ClinicalTrials.gov: NCT00098657/NCT00083889]
-
- Castellano D, Muro XG, Perez-Gracia JL, Gonzalez-Larriba JL, Abrio MV, Ruiz MA, et al. Patient-reported outcomes in a phase III, randomized study of sunitinib versus interferon-{alpha} as first-line systemic therapy for patients with metastatic renal cell carcinoma in a European population. Annals of Oncology 2009;20(11):1803-12. [ClinicalTrials.gov: NCT00098657/NCT00083889] - PMC - PubMed
-
- Cella D, Bushmakin AG, Cappelleri JC, Charbonneau C, Li JZ, Kim ST, et al. Health-related quality of life (HrQoL) in metastatic renal cell carcinoma (mRCC) patients receiving sunitinib (Su) or interferon (IFN)-alfa in a randomized phase III trial: updated geographic analysis. Annals of Oncology 2009;19(Suppl 8):189. [ClinicalTrials.gov: NCT00098657/NCT00083889]
NCT00117637 {published data only}
-
- Escudier B, Szczylik C, Demkow T, Staehler M, Rolland F, Negrier S, et al. Randomized phase II trial of the multi-kinase inhibitor sorafenib versus interferon (IFN) in treatment-naive patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18S):4501. [ClinicalTrials.gov: NCT00117637] - PubMed
-
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(8):1280-9. [ClinicalTrials.gov: NCT00117637] - PubMed
-
- Escudier. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(13):2305. [ClinicalTrials.gov: NCT00117637] - PubMed
-
- Felsch M, Zaim S, Dicken V, Lehmacher W, Scheuring UJ. Comparison of central and local serial CT assessments of metastatic renal cell carcinoma patients in a clinical phase IIB study. Acta Radiologica 2017;58(2):249-55. [ClinicalTrials.gov: NCT00117637] - PubMed
-
- Szczylik C, Demkow T, Staehler M, Rolland F, Negrier S, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon in patients with advanced renal cell carcinoma: final results. Journal of Clinical Oncology: ASCO annual meeting proceedings 2007;25(18 Suppl):Abstract 5025. [ClinicalTrials.gov: NCT00117637] - PubMed
NCT00126594 {published data only}
-
- Sorafenib tosylate with or without recombinant interferon alpha-2b in treating patients with metastatic kidney cancer. https://clinicaltrials.gov/ct2/show/NCT00126594. [ClinicalTrials.gov: NCT00126594]
NCT00334282 {published and unpublished data}
-
- Cella D, Pickard AS, Duh MS, Guerin A, Mishagina N, Antras L, et al. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomised phase III trial. European Journal of Cancer 2012;48(3):311-23. [ClinicalTrials.gov: NCT00334282] - PubMed
-
- Pickard AS, Cella D, Duh MS, Guerin A, Mishagina N, Antras L, et al. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomized phase III trial. In: Journal of Clinical Oncology. Vol. 29. 2011. [ClinicalTrials.gov: NCT00334282] - PubMed
-
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of Clinical Oncology 2010;28(6):1061-8. [ClinicalTrials.gov: NCT00334282] - PubMed
-
- Sternberg CN, Hawkins RE, Szczylik C, Davis ID, Wagstaff J, McCann L, et al. A randomized, double-blind phase III study (VEG105192) of pazopanib (paz) versus placebo (pbo) in patients with advanced/metastatic renal cell carcinoma (mRCC): updated safety results. Journal of Clinical Oncology 2011;29(7 Suppl 1):313. [ClinicalTrials.gov: NCT00334282]
-
- Sternberg CN, Hawkins RE, Szczylik C, Davis ID, Wagstaff J, McCann L, et al. Randomized, double-blind phase III study of pazopanib in patients with advanced/metastatic renal cell carcinoma (mRCC): final overall survival (OS) results. Annals of Oncology 2010;8:viii10. [ClinicalTrials.gov: NCT00334282]
NCT00420888 {published data only}
-
- Eisen T, Hedlund G, Forsberg G, Nordle O, Hawkins R. Baseline biomarker trend analysis of a randomized phase 2/3 study of naptumomab estafenatox plus IFN-a vs IFN-a in advanced renal cell carcinoma. European Journal of Cancer 2013;2:S645. [ClinicalTrials.gov: NCT00420888]
-
- Elkord E, Burt DJ, Sundstedt A, Nordle Ö, Hedlund G, Hawkins RE. Immunological response and overall survival in a subset of advanced renal cell carcinoma patients from a randomized phase 2/3 study of naptumomab estafenatox plus IFN-α versus IFN-α. Oncotarget 2015;6(6):4428-39. [ClinicalTrials.gov: NCT00420888] - PMC - PubMed
-
- Hawkins RE, Gore M, Shparyk Y, Bondar V, Gladkov O, Ganev T, et al. A randomized phase II/III study of naptumomab estafenatox + IFNα versus IFNα in renal cell carcinoma: final analysis with baseline biomarker subgroup and trend analysis. Clinical Cancer Research 2016;22(13):3172-81. [ClinicalTrials.gov: NCT00420888] - PubMed
-
- NCT00420888. ABR-217620/Naptumomab Estafenatox With Interferon-alpha (IFN-alpha) Compared to IFN-alpha Alone in Patients With Advanced Renal Cell Carcinoma. clinicaltrials.gov/show/NCT00420888 first received 11 January 2007. [ClinicalTrials.gov: NCT00420888]
NCT00609401 {published data only}
-
- Procopio G, Verzoni E, Bracarda S, Conti G, Guadalupi V, Ortega C, et al. Subgroup analysis and updated results of a randomized study comparing sorafenib plus interleukin-2 versus sorafenib alone as first-line treatment in metastatic renal cell carcinoma. Anticancer Research 2010;30(4):1461-2. [ClinicalTrials.gov: NCT00609401]
-
- Procopio G, Verzoni E, Bracarda S, Ricci S, Miceli R, Bertolini A, et al. A randomized, prospective, phase 2 study, with sorafenib (So) and interleukin-2 (IL-2) versus So alone as first line treatment in advanced renal cell cancer (RCC): ROSORC trial. European Journal of Cancer 2009;7(2-3):425. [ClinicalTrials.gov: NCT00609401]
-
- Procopio G, Verzoni E, Bracarda S, Ricci S, Ridolfi L, Sacco C, et al. Overall survival (OS) of sorafenib (So) plus interleukin-2 (IL-2) versus So alone in patients with treatment-naive metastatic renal cell carcinoma (mRCC): final update of the ROSORC trial. Journal of Clinical Oncology 2013;31(6 Suppl 1):356. [ClinicalTrials.gov: NCT00609401]
-
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. A randomized, open label, prospective study comparing the association between sorafenib (So) and interleukin-2 (IL-2) versus So alone in advanced untreated renal cell cancer (RCC): ROSORC trial. Journal of Clinical Oncology 2009;27(15S Pt 1):258. [ClinicalTrials.gov: NCT00609401]
-
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Overall survival for sorafenib plus interleukin-2 compared with sorafenib alone in metastatic renal cell carcinoma (mRCC): final results of the ROSORC trial. Annals of Oncology 2013;24(12):2967-71. [ClinicalTrials.gov: NCT00609401] - PubMed
NCT00619268 {published data only}
-
- Bay JO, Negrier S, Perol D, Gravis G, Chevreau C, Delva R, et al. Updated results on long-term overall survival (OS) of the French randomized phase II trial TORAVA in metastatic renal cell carcinoma (mRCC) patients. Journal of Clinical Oncology 2012;30(15 Suppl 1):4625. [ClinicalTrials.gov: NCT00619268]
-
- Escudier BJ, Negrier S, Gravis G, Chevreau C, Delva R, Bay J, et al. Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma? Results of the randomized TORAVA phase II trial. Journal of Clinical Oncology: ASCO annual meeting proceedings 2010;28(15 Suppl):Abstract 4516. [ClinicalTrials.gov: NCT00619268]
-
- Negrier S, Gravis G, Perol D, Chevreau C, Delva R, Bay JO, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncology 2011;12(7):673-80. [ClinicalTrials.gov: NCT00619268] - PubMed
NCT00631371 {published data only}
-
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer A, Escudier B. Randomized phase IIIb trial of temsirolimus and bevacizumab versus interferon and bevacizumab in metastatic renal cell carcinoma: results from intoract. Annals of Oncology 2012;9:ix14. [ClinicalTrials.gov: NCT00631371] - PubMed
-
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. Journal of Clinical Oncology 2014;32(8):752-9. [ClinicalTrials.gov: NCT00631371] - PubMed
NCT00719264 {published data only}
-
- Ravaud A, Bajetta E, Kay AC, Pelov D, Hollaender N, Rouyrre N, et al. Everolimus with bevacizumab versus interferon alfa-2a plus bevacizumab as first-line therapy in patients with metastatic clear cell renal cell carcinoma. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 28. 2010. [ClinicalTrials.gov: NCT00719264]
-
- Ravaud A, Barrios CH, Alekseev B, Tay MH, Agarwala SS, Yalcin S, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon alpha-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Annals of Oncology 2015;26(7):1378-84. [ClinicalTrials.gov: NCT00719264] - PubMed
-
- Ravaud A, Barrios CH, Alekseev BY, Tay MH, Agarwala SS, Yalcin S, et al. Randomized phase II study of first-line everolimus plus bevacizumab (E+B) versus interferon alpha-2a plus bevacizumab (I+B) in patients (pts) with metastatic renal cell carcinoma (mRCC): Record-2 final overall survival (OS) and safety results. Journal of Clinical Oncology: ASCO annual meeting proceedings 2013;31(15 Suppl 1):4576. [ClinicalTrials.gov: NCT00719264]
NCT00720941 {published and unpublished data}
-
- Beaumont JL, Diaz J, Deen KC, McCann L, Powles T, Hackshaw MD, et al. Q-TWiST analysis of patients with metastatic renal cell carcinoma (mRCC) randomized to pazopanib (PAZ) or sunitinib (SUN). Journal of Clinical Oncology: ASCO annual meeting proceedings 2014;32(15 Suppl 1):4581. [ClinicalTrials.gov: NCT00720941]
-
- Bjarnason GA, Kollmannsberger C, Ahmad Q, Dezzani L, Elmeliegy M, Han J, et al. Effects of pazopanib (PAZ) and sunitinib (SUN) dose modification on safety and efficacy in patients with metastatic renal cell carcinoma (mRCC) from COMPARZ. Kidney cancer 2018;2:S18. [ClinicalTrials.gov: NCT00720941]
-
- Bjarnason GA, Kollmannsberger CK, Ahmad Q, Dezzani L, Elmeliegy M, Han J, et al. Effects of pazopanib (PAZ) and sunitinib (SUN) dose modification on safety and efficacy in patients with metastatic renal cell carcinoma (mRCC) from COMPARZ. Journal of Clinical Oncology 2017;35(15 Suppl 1):4574. [ClinicalTrials.gov: NCT00720941]
-
- Cella D, Hackshaw MD, Diaz J, Huang C, Deen KC, Crescenzo R, et al. Quality of life (QoL) among patients with renal cell carcinoma (RCC) treated with pazopanib versus sunitinib in the COMPARZ study. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):346. [ClinicalTrials.gov: NCT00720941]
-
- Guo J, Jin J, Huang Y, Wang JW, Lim HY, Uemura H, et al. Comparison of PFS and safety for Asian compared to North American and European populations in the phase III trial of pazopanib versus sunitinib in patients with treatment-naive RCC (COMPARZ). Journal of Clinical Oncology 2013;31(6 Suppl 1):366. [ClinicalTrials.gov: NCT00720941]
NCT00732914 {published data only}
-
- Eichelberg C, Vervenne WL, De Santis M, Fischer von Weikersthal L, Goebell PJ, Lerchenmüller C, et al. SWITCH: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the treatment of metastatic renal cell cancer. European Urology 2015;68(5):837-47. [ClinicalTrials.gov: NCT00732914] - PubMed
-
- Fischer Von Weikersthal L, Goebell PJ, De Santis M, Lerchenmuller C, Zimmermann U, Bos MMEM, et al. SWITCH: A randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So) / sunitinib (Su) versus Su/So in the treatment of metastatic renal cell cancer (mRCC). Oncology Research and Treatment 2014;5:5. [ClinicalTrials.gov: NCT00732914] - PubMed
-
- Fischer Von Weikersthal L, Vervenne WL, Goebell PJ, Eichelberg C, Freier W, De Santis M, et al. Phase III randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So) followed by sunitinib (Su) versus sunitinib followed by sorafenib in patients with advanced / metastatic renal cell carcinoma without prior systemic therapy (SWITCH Study)-Safety interim analysis results. Onkologie 2012;6:237. [ClinicalTrials.gov: NCT00732914]
-
- Goebell PJ, Vervenne W, De Santis M, Von Weikersthal LF, Lerchenmuller CA, Zimmermann U, et al. Subgroup analyses of a randomized sequential open-label study (SWITCH) to evaluate efficacy and safety of sorafenib (So)/sunitinib (Su) versus Su/So in the treatment of metastatic renal cell cancer (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2014;32(15 Suppl 1):4567. [ClinicalTrials.gov: NCT00732914]
-
- Michel MS, Vervenne W, De Santis M, Von Weikersthal LF, Goebell PJ, Lerchenmueller J, et al. SWITCH: a randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So)/sunitinib (Su) versus Su/So in the treatment of metastatic renal cell cancer (mRCC). Journal of Clinical Oncology 2014;32(4 Suppl 1):393. [ClinicalTrials.gov: NCT00732914]
NCT00738530 {published data only}
-
- Bajetta E, Ravaud A, Bracarda S, Negrier S, Szczylik C, Bellmunt Molins J, et al. Efficacy and safety of first-line bevacizumab (BEV) plus interferon-a2a (IFN) in patients (pts) >65 years with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2008;26(15S pt I):273. [ClinicalTrials.gov: NCT00738530]
-
- Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. Journal of Clinical Oncology 2010;28(13):2144-50. [ClinicalTrials.gov: NCT00738530] - PubMed
-
- Escudier B, Bellmunt J, Negrier S, Melichar B, Bracarda S, Ravaud A, et al. Final results of the phase III, randomized, double-blind AVOREN trial of first-line bevacizumab (BEV) + interferon alfa-2a (IFN) in metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2009;27(15S Pt 1):239. [ClinicalTrials.gov: NCT00738530]
-
- Escudier B, Koralewski P, Pluzanska A, Ravaud A, Bracarda S, Szczylik C, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-a2a vs placebo/interferon-α2a as first-line therapy in metastatic renal cell carcinoma. Journal of Clinical Oncology: ASCO annual meeting proceedings 2007;25(18 Suppl):Abstract 3. [ClinicalTrials.gov: NCT00738530]
-
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370(9605):2103-11. [ClinicalTrials.gov: NCT00738530] - PubMed
NCT00903175 {published data only}
-
- Hsieh J, Chen D, Wang P, Chen Y, Marker M, Patel P, et al. Differential overall survival (OS) results in RECORD-3 study based on three distinct mRCC molecular subgroups classified by BAP1 and/or PBRM1 mutations. Journal of Clinical Oncology 2016;34(2 Suppl 1):561. [ClinicalTrials.gov: NCT00738530]
-
- Hsieh J, Chen D, Wang P, Chen YB, Mahtab M, Pate P, et al. Differential overall survival results in RECORD-3 study based on three distinct clear cell metastatic renal cell carcinoma molecular subgroups classified by BAP1 and/ or PBRM1 mutations. BJU International 2016;118 Suppl 5:10. [ClinicalTrials.gov: NCT00738530]
-
- Hsieh JJ, Chen D, Wang PI, Chen YB, Redzematovic A, Marker M, et al. Identification of efficacy biomarkers in a large metastatic renal cell carcinoma (mRCC) cohort through next-generation sequencing (NGS): results from RECORD-3. BJU International 2015;5:13. [ClinicalTrials.gov: NCT00738530]
-
- Hsieh JJ, Chen D, Wang PI, Marker M, Redzematovic A, Chen YB, et al. Genomic biomarkers of a randomized trial comparing first-line everolimus and sunitinib in patients with metastatic renal cell carcinoma. European Urology 2017;71(3):405-14. [ClinicalTrials.gov: NCT00738530] - PMC - PubMed
-
- Knox JJ, Barrios CH, Kim TM, Cosgriff T, Srimuninnimit V, Pittman KB, et al. Final overall survival analysis for the RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC (mRCC). Journal of Clinical Oncology 2015;33(15 Suppl 1):4554. [ClinicalTrials.gov: NCT00738530]
NCT00920816 {published data only}
-
- Hutson TE, Al-Shukri S, Stus VP, Lipatov ON, Shparyk Y, Bair AH, et al. Axitinib versus sorafenib in first-line metastatic renal cell carcinoma: overall survival from a randomized phase III trial. Clinical Genitourinary Cancer 2017;15(1):72-6. [ClinicalTrials.gov: NCT00920816] - PubMed
-
- Hutson TE, Al-Shukri S, Stus VP, Lipatov ON, Shparyk Y, Bair AH, et al. Overall survival analysis from a randomised phase III trial of axitinib vs sorafenib as first-line therapy in patients with metastatic renal cell carcinoma. European Journal of Cancer 2015;51:476-77. [ClinicalTrials.gov: NCT00920816]
-
- Hutson TE, Gallardo J, Lesovoy V, Al-Shukri S, Stus V, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2013;31(6 Suppl 1):1287-94. [ClinicalTrials.gov: NCT00920816] - PubMed
-
- Sheng X, Bi F, Ren X, Cheng Y, Wang J, Rosbrook B, et al. First-line axitinib versus sorafenib in Asian patients with metastatic renal cell carcinoma (mRCC): subgroup analysis of data from a phase III trial. Annals of Oncology 2017;28:x82. [ClinicalTrials.gov: NCT00920816] - PubMed
-
- Sheng X. First-line axitinib versus sorafenib in Asian patients with metastatic renal cell carcinoma: exploratory subgroup analyses of phase III data. Future Oncology 2019;15(1):3267-77. [ClinicalTrials.gov: NCT00920816] - PubMed
NCT00979966 {published data only}
-
- Bergmann L, Grunwald V, Maute L, Grimm MO, Weikert S, Schleicher J, et al. A prospective randomized phase-II trial with temsirolimus vs. sunitinib in non-clear renal cell carcinoma. A study of the CESAR central european society for anticancer drug research-EWIV and interdisciplinary renal cell carcinoma group of the German cancer society (IAGN). European Journal of Cancer 2015;3:517. [ClinicalTrials.gov: NCT00979966] - PubMed
-
- Bergmann L, Grunwald V, Maute L, Grimm MO, Weikert S, Schleicher J, et al. A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society. Oncology Research and Treatment 2020;43(7-8):333-9. [ClinicalTrials.gov: NCT00979966] - PubMed
NCT01024920 {published data only}
-
- Eisen T, Loembe AB, Shparyk Y, MacLeod N, Jones RJ, Mazurkiewicz M, et al. A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-year results. British Journal of Cancer 2015;113(8):1140-7. [ClinicalTrials.gov: NCT01024920] - PMC - PubMed
-
- Eisen T, Shparyk Y, Jones R, MacLeod NJ, Temple G, Finnigan H, et al. Phase II efficacy and safety study of nintedanib versus sunitinib in previously untreated renal cell carcinoma (RCC) patients. Journal of Clinical Oncology 2013;31(15 Suppl 1):4506. [ClinicalTrials.gov: NCT01024920]
-
- Eisen T, Shparyk Y, MacLeod NJ, Wallenstein G, Temple G, Khder Y, et al. Effects of nintedanib (BIBF 1120) on QTc interval in previously untreated patients with renal cell cancer (RCC): results from an open-label, phase II study. In: Journal of Clinical Oncology (Conference). Vol. 30. 2012. [ClinicalTrials.gov: NCT01024920]
-
- Eisen T, Shparyk Y, Macleod N, Jones R, Wallenstein G, Temple G, et al. Effect of small angiokinase inhibitor nintedanib (BIBF 1120) on QT interval in patients with previously untreated, advanced renal cell cancer in an open-label, phase II study. Investigational New Drugs 2013;31(5):1283-93. [ClinicalTrials.gov: NCT01024920] - PubMed
NCT01030783 {published data only}
-
- Cella D, Ivanescu C, Skaltsa K, Casamayor M, Strahs AL, Esteves B, et al. Treatment benefit of tivozanib hydrochloride versus sorafenib on health-related quality of life (HRQoL) among patients (pts) with advanced/metastatic renal cell carcinoma (mRCC): TIVO-1 study results. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):355. [ClinicalTrials.gov: NCT01030783]
-
- Eisen TQG, Sternberg CN, Tomczak P, Harza M, Jinga V, Esteves B, et al. Detailed comparison of the safety of tivozanib versus sorafenib in patients with advanced/metastatic renal cell carcinoma (mRCC) from a phase 3 trial. Annals of Oncology 2012;9:ix262-3. [ClinicalTrials.gov: NCT01030783]
-
- Hutson T, Nosov D, Tomczak P, Bondarenko I, Lipatov ON, Sternberg CN, et al. Tivozanib vs sorafenib targeted therapy for advanced renal cell carcinoma: final results of a phase III trial (901) and efficacy results of a 2nd line tivozanib extension study (902). Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4557. [ClinicalTrials.gov: NCT01030783]
-
- Hutson TE, Nosov D, Eisen T, Lipatov ON, Tomczak P, Alyasova A, et al. Subgroup analyses of a phase III trial comparing tivozanib hydrochloride versus sorafenib as initial targeted therapy for patients (pts) with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2013;31(6 Suppl 1):354. [ClinicalTrials.gov: NCT01030783] - PMC - PubMed
-
- Hutson TE, Nosov D, Harza M, Esteves B, Strahs AL, Berkenblit A, et al. Fewer dose adjustments with tivozanib vs. sorafenib in the phase III TIVO-1 study in advanced renal cell carcinoma (RCC). Urologe 2013;52(1 Suppl 1):89-90. [ClinicalTrials.gov: NCT01030783]
NCT01064310 {published and unpublished data}
-
- Cella D, Kaiser K, Beaumont J, Diaz J, McCann L, Mehmud F, et al. Quality of life (QoL) among renal cell carcinoma (RCC) patients in a randomized double blind cross-over patient preference study of pazopanib (P) versus sunitinib (S). Annals of Oncology 2012;9:ix261-2. [ClinicalTrials.gov: NCT01064310]
-
- Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. Journal of Clinical Oncology 2014;32(14):1412-8. - PubMed
-
- Lai JS, Beaumont JL, Diaz J, Khan S, Cella D. Validation of a short questionnaire to measure symptoms and functional limitations associated with hand-foot syndrome and mucositis in patients with metastatic renal cell carcinoma. Cancer 2016;122(2):287-95. - PubMed
NCT01108445 {published data only}
-
- Armstrong AJ, Broderick S, Eisen T, Stadler WM, Jones RJ, Garcia JA, et al. Final clinical results of a randomized phase II international trial of everolimus vs. Sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). Journal of Clinical Oncology 2015;33(15 Suppl 1):4507. [ClinicalTrials.gov: NCT01064310]
-
- Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncology 2016;17(3):378-88. [ClinicalTrials.gov: NCT01064310] - PMC - PubMed
-
- Armstrong AJ, Halabi S, Eisen T, Stadler WM, Jones RR, Vaishampayan UN, et al. ASPEN: a randomized phase II trial of everolimus versus sunitinib in patients with metastatic non-clear cell renal cell carcinoma. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 31. 2013. [ClinicalTrials.gov: NCT01064310]
-
- Armstrong AJ, Nixon AB, Carmack A, Eisen T, Stadler WM, Jones RJ, et al. Angiokines associated with outcomes after sunitinib or everolimus treatment in patients with non-clear cell renal cell carcinoma. Clinical Cancer Research 2021;16:16. [ClinicalTrials.gov: NCT01064310] - PubMed
NCT01274273 {published data only}
-
- Donskov F, Jensen NV, Smidt-Hansen T, Brondum L, Geertsen PF. A randomized phase II trial of interleukin-2/interferon-alpha plus bevacizumab versus interleukin-2/interferon-alpha in metastatic renal cell carcinoma (mRCC): results from the Danish renal cancer group (DARENCA) study 1. Journal of Clinical Oncology 2016;34(15 Suppl):4563. [ClinicalTrials.gov: NCT01274273] - PubMed
-
- Donskov F, Jensen NV, Smidt-Hansen T, Brøndum L, Geertsen P. A randomized phase II trial of interleukin-2 and interferon-α plus bevacizumab versus interleukin-2 and interferon-α in metastatic renal-cell carcinoma (mRCC): results from the Danish renal cancer group (DaRenCa) study-1. Acta Oncologica 2018;57(5):589-94. [ClinicalTrials.gov: NCT01274273] - PubMed
NCT01392183 {published data only}
-
- Rini BI, Gruenwald V, Fishman MN, Melichar B, Ueda T, Bair AH, et al. Axitinib with or without dose titration for first-line metastatic renal cell carcinoma (mRCC): unblinded results from a randomized phase II study. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 31. 2013. [ClinicalTrials.gov: NCT01392183]
-
- Rini BI, Melichar B, Ueda T, Fishman MN, Pithavala Y, Chen Y, et al. Randomised phase II trial of axitinib with or without dose titration in first-line metastatic renal cell carcinoma (mRCC): efficacy and pharmacokinetic (PK) analyses. European Journal of Cancer 2013;2:S657. [ClinicalTrials.gov: NCT01392183]
-
- Tannir NM, Msaouel P, Ross JA, Devine CE, Chandramohan A, Gonzalez GM, et al. Temsirolimus versus pazopanib (TemPa) in patients with advanced clear-cell renal cell carcinoma and poor-risk features: a randomized phase II trial. European Urology Oncology 2019;3(5):687-694. [ClinicalTrials.gov: NCT01392183] - PMC - PubMed
-
- Tannir NM, Msaouel P, Ross JA, Devine CE, Chandramohan A, Gonzalez GM, et al. Temsirolimus versus pazopanib (TemPa) in patients with advanced clear-cell renal cell carcinoma and poor-risk features: a randomized phase II trial. European Urology Oncology 2020;3(5):687-94. [ClinicalTrials.gov: NCT01392183] - PMC - PubMed
-
- Tannir NM, Msaouel P, Ross JA, Devine CE, Chandramohan A, Gonzalez GM, et al. Temsirolimus versus pazopanib (TemPa) in patients with advanced clear-cell renal cell carcinoma and poor-risk features: a randomized phase II trial. European Urology Oncology 2020;3(5):687-94. [ClinicalTrials.gov: NCT01392183] - PMC - PubMed
NCT01481870 {published data only}
-
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as first-line therapy followed by sorefenib and sunitinib for patients with metastatic renal cell carcinoma (RCC) with clear cell histology: a multicenter randomized trial, CROSS-J-RCC. Journal of Clinical Oncology 2017;35(6):469. [ClinicalTrials.gov: NCT01481870]
-
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as first-line therapy for patients with metastatic renal cell carcinoma with favorable or intermediate MSKCC risk factors: a multicenter randomized trial, CROSS-J-RCC. Journal of Clinical Oncology 2014;32(4 Suppl 1):502. [ClinicalTrials.gov: NCT01481870]
-
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as initial targeted therapy for mCC-RCC with favorable/intermediate risk: multicenter randomized trial CROSS-J-RCC. Clinical Genitourinary Cancer 2020;18(4):e374-85. [ClinicalTrials.gov: NCT01481870] - PubMed
NCT01613846 {published data only}
-
- Gschwend J, Beeker A, De Santis M, Brehmer B, Zaiss MR, Schirrmacher-Memmel S, et al. Phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the treatment of advanced/metastatic renal cell carcinoma (SWITCH-2 study). In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 31. 2013. [ClinicalTrials.gov: NCT01613846]
-
- Retz M, Bedke J, Bogemann M, Grimm MO, Zimmermann U, Muller L, et al. SWITCH II: phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of advanced or metastatic renal cell carcinoma (AUO AN 33/11). European Journal of Cancer 2019;107:37-45. [ClinicalTrials.gov: NCT01613846] - PubMed
-
- Retz M, Bedke J, Herrmann E, Grimm MO, Zimmermann U, Muller L, et al. Phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of metastatic renal cell carcinoma (SWITCH-II). Annals of Oncology 2017;28 Suppl 5:v295. [ClinicalTrials.gov: NCT01613846] - PubMed
-
- Rexer H. First line therapy of advanced or metastasized renal cell carcinoma: open, randomized phase III sequence study to examine the effectiveness and tolerance of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the first-line treatment of patients with advanced or metastasized renal cell carcinoma (SWITCH-2-AN 33/11).. Urologe - Ausgabe A 2012;51(5):724-6. [ClinicalTrials.gov: NCT01613846] - PubMed
-
- Rexer H. First-line therapy of advanced or metastasized renal cell carcinoma: phase III, open, randomized sequence study to examine efficacy and tolerance of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the first-line treatment of patients with advanced or metastasized renal cell carcinoma (SWITCH-2 - AN 33/11). Der Urologe 2014;53(5):735-8. [ClinicalTrials.gov: NCT01613846] - PubMed
NCT01835158 {published data only}
-
- Alba SO, Jose MB, Silvia FC. Cabozantinib for the treatment of advanced renal cell carcinoma in treatment-naive adults. European Journal of Clinical Pharmacy 2019;21(3):160-3. [ClinicalTrials.gov: NCT01835158]
-
- Chen RC, Choueiri TK, Feuilly M, Meng J, Lister J, Marteau F, et al. Quality-adjusted survival with first-line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance). Cancer 2020;126(24):5311-18. [ClinicalTrials.gov: NCT01835158] - PMC - PubMed
-
- Chen RC, Feuilly M, Meng J, Lister J, Marteau F, Morris MJ, et al. Quality-adjusted time without symptoms or toxicity (Q-TWiST): analysis of cabozantinib (Cabo) vs sunitinib (Sun) in patients with advanced renal cell carcinoma (aRCC) of intermediate or poor risk (Alliance A031203). Journal of Clinical Oncology 2018;36(15 Suppl 1):4556. [ClinicalTrials.gov: NCT01835158]
-
- Choueiri TK, Halabi S, Sanford B, Hahn O, Michaelson MD, Walsh M, et al. CABOzantinib versus SUNitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: results from ALLIANCE A031203 trial. Annals of Oncology 2016;27 Suppl 6:vi566. [ClinicalTrials.gov: NCT01835158] - PMC - PubMed
-
- Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. Journal of Clinical Oncology 2017;35(6):591-7. [ClinicalTrials.gov: NCT01835158] - PMC - PubMed
NCT01984242 {published data only}
-
- Atkins MB, McDermott DF, Powles T, Motzer RJ, Rini BI, Fong L, et al. IMmotion150: a phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun). Journal of Clinical Oncology 2017;35(15):4505. [ClinicalTrials.gov: NCT01984242]
-
- Grunwald V, McDermott DF, Atkins M, Motzer R, Rini B, Escudier B, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) vs sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Oncology Research and Treatment 2017;40 Suppl 3:113-4. [ClinicalTrials.gov: NCT01984242]
-
- McDermott DF, Atkins MB, Motzer RJ, Rini BI, Escudier BJ, Fong L, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Journal of Clinical Oncology 2017;35(6):431. [ClinicalTrials.gov: NCT01984242]
-
- McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nature Medicine 2018;24(6):749-57. [ClinicalTrials.gov: NCT01984242] - PMC - PubMed
-
- Pal S, Powles T, McDermott D, Rini B, Motzer R, Atkins M, et al. IMmotion150: novel radiological endpoints and updated data from a randomized phase II trial investigating atezolizumab with or without bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma. Kidney Cancer 2018;2 Suppl 1:S22-3. [ClinicalTrials.gov: NCT01984242]
NCT02231749 {published data only}
-
- Albiges L, Tannir N, Burotto M, McDermott DF, Plimack ER, Barthelemy P, et al. Nivolumab + ipilimumab (N+I) vs sunitinib (S) for first-line treatment of advanced renal cell carcinoma (aRCC) in CheckMate 214: 4-year follow-up and subgroup analysis of patients (pts) without nephrectomy. Annals of Oncology 2020;31 Suppl 4:S559-60. [ClinicalTrials.gov: NCT02231749]
-
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. First-line nivolumab plus ipilimumab versus sunitinib in patients without nephrectomy and with an evaluable primary renal tumor in the CheckMate 214 trial. European Urology 2021;81(3):266-271. [ClinicalTrials.gov: NCT02231749] - PMC - PubMed
-
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5(6):e001079. [ClinicalTrials.gov: NCT02231749] - PMC - PubMed
-
- Ali S, Camarero J, Hennik PV, Bolstad B, Sommerfelt Gronvold M, Syvertsen C, et al. European Medicines Agency extension of indication to include the combination immunotherapy cancer drug treatment with nivolumab (Opdivo) and ipilimumab (Yervoy) for adults with intermediate/poor-risk advanced renal cell carcinoma. ESMO Open 2020;5(6):e000798. [ClinicalTrials.gov: NCT02231749] - PMC - PubMed
-
- Branchoux S, Négrier S, Peretti C, Malcolm B, May J, Marié L, et al. PCN214 cost-effectiveness analysis of nivolumab in combination with ipilimumab versus sunitinib for the first-line treatment of intermediate- to poor-risk advanced renal cell carcinoma in France. In: Value in Health. Vol. 22. 2019:S477. [ClinicalTrials.gov: NCT02231749]
NCT02420821 {published data only}
-
- Atkins MB, Rini BI, Motzer RJ, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes from the phase III randomized IMmotion151 trial: atezolizumab bevacizumab versus sunitinib in treatment-naive metastatic renal cell carcinoma. Clinical Cancer Research 2020;26(11):2506-14. [ClinicalTrials.gov: NCT02420821] - PMC - PubMed
-
- Escudier B, Motzer RJ, Rini BI, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes (PROs) in IMmotion151: atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun) in treatment (tx) naive metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2018;36(15):4511. [ClinicalTrials.gov: NCT02420821]
-
- Grullich C, Motzer RJ, Powles T, Atkins M, Escudier BJ, McDermott D, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). Oncology Research and Treatment 2018;41 Suppl 4:41-2. [ClinicalTrials.gov: NCT02420821]
-
- Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekseev BY, et al. Final overall survival and molecular analysis in IMmotion151, apPhase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. In: JAMA Oncology. Vol. 23. 2021:23. [ClinicalTrials.gov: NCT02420821] - PMC - PubMed
-
- Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2018;36(6):578. [ClinicalTrials.gov: NCT02420821]
NCT02684006 {published data only}
-
- Albiges L, Rini BI, Haanen JB, Motzer RJ, Kollmannsberger CK, Negrier S, et al. Primary renal tumour shrinkage in patients (pts) who did not undergo upfront cytoreductive nephrectomy (uCN): subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab 1 axitinib (A 1 Ax) vs sunitinib (S) for advanced renal cell carcinoma (aRCC). Annals of Oncology 2019;30 Suppl 5:v359-60. [ClinicalTrials.gov: NCT02684006]
-
- Bilen MA, Rini BI, Voss MH, Larkin J, Haanen JB, Albiges L, et al. Association of neutrophil-to-lymphocyte ratio with efficacy of first-line avelumab plus axitinib vs. sunitinib in patients with advanced renal cell carcinoma enrolled in the phase 3 JAVELIN renal 101 trial. Clinical Cancer Research 2021;17:17. [ClinicalTrials.gov: NCT02684006] - PMC - PubMed
-
- Choueiri TK, Larkin JM, Pal SK, Motzer RJ, Venugopal B, Alekseev BY, et al. Efficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (sRCC): subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A + Ax) vs sunitinib (S). Annals of Oncology 2019;30 Suppl 5:v361. [ClinicalTrials.gov: NCT02684006]
-
- Choueiri TK, Motzer RJ, Campbell MT, Alekseev BY, Uemura M, Kollmannsberger CK, et al. Subgroup analysis from JAVELIN renal 101: outcomes for avelumab plus axitinib (A + Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 2019;37(7):544. [ClinicalTrials.gov: NCT02684006]
-
- Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Annals of Oncology 2020;31(8):1030-1039. [ClinicalTrials.gov: NCT02684006] - PMC - PubMed
NCT02761057 {published data only}
-
- NCT02761057. Cabozantinib s-malate, crizotinib, volitinib, or sunitinib malate in treating patients with locally advanced or metastatic kidney cancer. clinicaltrials.gov/show/NCT02761057 first received 4 May 2016. [ClinicalTrials.gov: NCT02761057]
-
- Pal SK, Tangen C, Thompson IM, Balzer-Haas N, George DJ, Heng DY, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021;397(10275):695-703. [ClinicalTrials.gov: NCT02761057] - PMC - PubMed
-
- Pal SK, Tangen C, Thompson IM, Haas NB, George DJ, Heng DY, et al. Sunitinib versus cabozantinib, crizotinib or savolitinib in metastatic papillary renal cell carcinoma (pRCC): results from the randomized phase II SWOG 1500 study. Journal of Clinical Oncology 2021;39(6 Suppl):270. [ClinicalTrials.gov: NCT02761057]
-
- Pal SK, Tangen C, Thompson IM, Haas NB, George DJ, Heng DY, et al. Sunitinib versus cabozantinib, crizotinib or savolitinib in metastatic papillary renal cell carcinoma (pRCC): results from the randomized phase II SWOG 1500 study. Journal of Clinical Oncology 2021;39(6 Suppl):270. [ClinicalTrials.gov: NCT02761057]
-
- Pal SK, Tangen CM, Thompson IM, Shuch BM, Haas NB, George DJ, et al. A randomized, phase II efficacy assessment of multiple MET kinase inhibitors in metastatic papillary renal carcinoma (PRCC): SWOG S1500. In: Journal of Clinical Oncology (Conference). Vol. 35. 2017. [ClinicalTrials.gov: NCT02761057]
NCT02811861 {published data only}
-
- Choueiri TK, Eto M, Kopyltsov E, Rha SY, Porta CG, Motzer R, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Annals of Oncology 2021;32 Suppl 5:S683-5. [ClinicalTrials.gov: NCT02811861]
-
- Gruenwald V, Powles T, Choueiri TK, Hutson TE, Porta C, Eto M, et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: study design and rationale. Future Oncology 2019;15(9):929-41. [ClinicalTrials.gov: NCT02811861] - PubMed
-
- Gruenwald V, Powles T, Kopyltsov E, Kozlov V, Alonso Gordoa T, Eto M, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (L) + pembrolizumab (P) and sunitinib (S) treatment arms. Oncology Research and Treatment 2021;44 Suppl 2:25. [ClinicalTrials.gov: NCT02811861]
-
- Gruenwald V, Powles T, Kopyltsov E, Kozlov V, Gordoa TA, Eto M, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. Journal of Clinical Oncology 2021;39(15 Suppl):4560. [ClinicalTrials.gov: NCT02811861]
-
- Hutson TE, Choueiri TK, Motzer RJ, Rha SY, Alyasova A, Merchan JR, et al. Post hoc analysis of the CLEAR study in advanced renal cell carcinoma (RCC): effect of subsequent therapy on survival outcomes in the lenvatinib (LEN) + everolimus (EVE) versus sunitinib (SUN) treatment arms. Journal of Clinical Oncology 2021;39(15 Suppl):4562. [ClinicalTrials.gov: NCT02811861]
NCT02853331 {published data only}
-
- Correction to Lancet Oncol 2020; 21: 1563–73 (The Lancet Oncology (2020) 21(12) (1563–1573), (S1470204520304368), (10.1016/S1470-2045(20)30436-8)). Lancet Oncology 2020;21(12):e553. [ClinicalTrials.gov: NCT02853331]
-
- Gafanov R, Powles TB, Bedke J, Stus V, Waddell TS, Nosov D, et al. Subsequent therapy following pembrolizumab + axitinib or sunitinib treatment for advanced renal cell carcinoma (RCC) in the phase III KEYNOTE-426 study. Annals of Oncology 2021;32 Suppl 5:S694. [ClinicalTrials.gov: NCT02853331]
-
- Kondoh CN, Bae WK, Tamada S, Matsubara N, Lee HJ, Mizuno R, et al. Pembrolizumab plus axitinib (pembro + axi) vs sunitinib in metastatic renal cell carcinoma (mRCC) outcomes of the KEYNOTE-426 study in patients from eastern Asia. Annals of Oncology 2020;31(Suppl 6):1319. [ClinicalTrials.gov: NCT02853331]
-
- Plimack ER, Powles T, Bedke J, Pouliot F, Stus V, Waddell T, et al. Outcomes for patients in the pembrolizumab+axitinib arm with advanced renal cell carcinoma (RCC) who completed two years of treatment in the phase III KEYNOTE-426 study. Journal of Clinical Oncology 2021;39(6 Suppl):327. [ClinicalTrials.gov: NCT02853331]
-
- Plimack ER, Rini BI, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy formetastatic renal cell carcinoma: outcomes in the combined IMDC intermediate-/poor-risk and sarcomatoid subgroups of the phase 3 Keynote-426 study. Asia-Pacific Journal of Clinical Oncology 2019;15:33-4. [ClinicalTrials.gov: NCT02853331]
NCT03141177 {published data only}
-
- Apolo AB, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Basso U, et al. Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Journal of Clinical Oncology 2021;39(15 Suppl):4553. [ClinicalTrials.gov: NCT03141177]
-
- Basso U, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Shah AY, et al. Nivolumab plus cabozantinib (N+C) vs sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Tumori Journal 2021;107(2 Suppl):42-3. [ClinicalTrials.gov: NCT03141177]
-
- Bedke J, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Basso U, et al. Nivolumab plus cabozantinib (N+C) vs sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Oncology Research and Treatment 2021;44 Suppl 2:24-5. [ClinicalTrials.gov: NCT03141177]
-
- Cella D, Choueiri TK, Blum SI, Ejzykowicz F, Hamilton M, Zhang J, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma (aRCC) treated with first-line nivolumab plus cabozantinib versus sunitinib: the CheckMate 9ER trial. Journal of Clinical Oncology 2021;39(6 Suppl):285. [ClinicalTrials.gov: NCT03141177]
-
- Cella D, Motzer RJ, Suarez C, Blum SI, Ejzykowicz F, Hamilton M, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncology 2022;23(2):292-303. [ClinicalTrials.gov: NCT03141177] - PMC - PubMed
References to studies excluded from this review
Aass 2005 {published data only}
-
- Aass N, De Mulder PH, Mickisch GH, Mulders P, Oosterom AT, Poppel H, et al. Randomized phase II/III trial of interferon alfa-2a with and without 13-cis-retinoic acid in patients with progressive metastatic renal cell carcinoma: the European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group (EORTC 30951). Journal of Clinical Oncology 2005;23(18):4172-8. - PubMed
Abdel 2018 {published data only}
-
- Abdel Hakim AM, Nouira Y, Taran R, Amokrane D, Ghosn M, Larbaoui B, et al. Everolimus as first-line or after cytokine therapy in patients with metastatic recurrent and/or unresectable renal cell carcinoma (RCC) (EVERMORE). Annals of Oncology 2018;29 Suppl 8:viii316.
Adler 1987 {published data only}
-
- Adler A, Gillon G, Lurie H, Shaham J, Loven D, Shachter Y, et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono-immuno- versus hormonotherapy. Preliminary report of immunological and clinical aspects. Journal of Biological Response Modifiers 1987;6(6):610-24. - PubMed
Amin 2018 {published data only}
Amin 2018a {published data only}
Atkins 1991 {published data only}
-
- Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Aronson FR, et al. Randomized phase II trial of high dose IL-2 either alone or in combination with interferon alpha 2B in advanced renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. 1991. - PubMed
Atkins 1993 {published data only}
-
- Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Fink KI, et al. Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. Journal of Clinical Oncology 1993;11(4):661-70. - PubMed
Atzpodien 1997 {published data only}
-
- Atzpodien J, Kirchner H, Franzke A, Wandert T, Probst M, Buer J, et al. Results of a randomized clinical trial comparing SC interleukin-2, SC alpha-2a-interferon, and IV bolus 5-fluorouracil against oral tamoxifen in progressive metastatic renal cell carcinoma patients. In: Proceedings of The American Society of Clinical Oncology. Vol. 16. 1997:326a, Abstract 1164.
Atzpodien 1997a {published data only}
-
- Atzpodien J, Kirchner H, Franzke A. A randomized clinical trial comparing sc interleukin-2, sc alpha-2a-interferon, and iv bolus 5-flurouracil against oral tamoxifen in progressive metastatic renal cell carcinoma patients. European Journal of Cancer 1997;33:S8, A151.
Atzpodien 1999 {published data only}
-
- Atzpodien J, Kirchner H, Bergmann L, Oberneder R, Jonas U, Ganser A. 13cis-retinoic acid, IFN-alpha, IL-2 and chemotherapy in advanced renal cell carcinoma: results of a prospectively randomized trial of the German Cooperative Renal Carcinoma Immunotherapy Group (DGCIN). In: Proceedings of The American Society of Clinical Oncology. Vol. 18. 1999:448a, Abstract 1727.
Atzpodien 2001 {published data only}
Atzpodien 2004 {published data only}
-
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Journal of Clinical Oncology 2004;22(7):1188-94. - PubMed
Atzpodien 2006 {published data only}
-
- Atzpodien J, Kirchner H, Rebmann U, Soder M, Gertenbach U, Siebels M, et al. Interleukin-2/interferon-alpha2a/13-retinoic acid-based chemoimmunotherapy in advanced renal cell carcinoma: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). British Journal Of Cancer 2006;95(4):463-9. - PMC - PubMed
Barrios 2009 {published data only}
-
- Barrios CH, Hernandez-Barajas D, Brown MP, Lee SH, Fein L, Liu JH, et al. Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma (mRCC): preliminary results. European Journal of Cancer, Supplement 2009;7(2-3):429-30. - PubMed
Beaumont 2009 {published data only}
-
- Beaumont J, Cella D, Hutson T, Bracarda S, Grunwald V, Thompson J, et al. Patient-reported outcomes in a randomized trial of everolimus with metastatic renal cell carcinoma patients. Journal of Clinical Oncology 2009;1:e17516.
Beaumont 2011 {published data only}
-
- Beaumont JL, Butt Z, Baladi J, Motzer RJ, Haas T, Hollaender N, et al. Patient-reported outcomes in a phase III study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist 2011;16(5):632-40. - PMC - PubMed
Berg 1998 {published data only}
-
- Berg WJ, Bukowski R, Thompson JA, Nemunaitis J, Murphy B, Ellerhorst J, et al. A randomized phase II trial of recombinant human interleukin-12 (IL-12) versus interferon alpha-2A (IFN) in advanced renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 17. 1998:318a, Abstract 1226.
Bex 2017 {published data only}
-
- Bex A, Mulders P, Jewett MA, Wagstaff J, Van Velthoven R, Laguna Pes PM, et al. Immediate versus deferred cytoreductive nephrectomy (CN) in patients with synchronous metastatic renal cell carcinoma (mRCC) receiving sunitinib (EORTC 30073 SURTIME). Annals of Oncology 2017;28 Suppl 5:v622. - PMC - PubMed
Boccardo 1998 {published data only}
-
- Boccardo F, Rubagotti A, Canobbio L, Galligioni E, Sorio R, Lucenti A, et al. Interleukin-2, interferon-alpha and interleukin-2 plus interferon-alpha in renal cell carcinoma. A randomized phase II trial. Tumori Journal 1998;84(5):534-9. - PubMed
Bracarda 2007 {published data only}
-
- Bracarda S, Porta C, Boni C. Randomized prospective phase II trial of two schedules of sorafenib daily and interferon-a2a in metastatic renal cell carcinoma (RAPSODY): GOIRC Study 0681. Journal Of Clinical Oncology 2007;25 Suppl:Abstract 5100.
Buckley 2019 {published data only}
-
- Buckley HL, Collinson FJ, Ainsworth G, Poad H, Flanagan L, Katona E et al. PRISM protocol: a randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. BMC Cancer 2019;19(1):1102. - PMC - PubMed
Cella 2016 {published data only}
-
- Cella D, Gruenwald V, Nathan PD, Doan J, Dastani H, Taylor F, et al. Quality of life (QoL) and overall survival (OS) in patients (pts) with advanced clear-cell renal cell carcinoma (aRCC) treated with nivolumab (NIVI) vs. everolimus (EVE) in the phase III CheckMate 025 study. Journal of Clinical Oncology: ASCO annual meeting proceedings 2016;34(15 Suppl):4549.
Choueiri 2017 {published data only}
-
- Choueiri TK, Jakacki R, Ghiorghiu D, Haddad V, Kohlmann A, Frigault MM, et al. Savolitinib versus sunitinib in patients with MET-driven, unresectable and locally advanced or metastatic papillary renal cell carcinoma: SAVOIR, a randomised, phase III trial. Annals of Oncology 2017;28:v328.
Choueiri 2020 {published data only}
Choueiri 2020a {published data only}
-
- Choueiri TK, Heng DY, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, et al. SAVOIR: a phase III study of savolitinib versus sunitinib in pts with MET-driven papillary renal cell carcinoma (PRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15 Suppl):5002.
Cirkel 2016 {published data only}
-
- Cirkel GA, Hamberg P, Sleijfer S, Loosveld O, Dercksen W, Los M, et al. A randomized phase II study to compare the efficacy of upfront bi-monthly rotations between pazopanib (PAZ) and everolimus (EVE) versus sequential treatment of first-line PAZ and second-line EVE until progression in patients with metastatic clear cell renal cell cancer (ccRCC) (ROPETAR trial). Journal of Clinical Oncology 2016;34(15):4550.
Cirkel 2017 {published data only}
-
- Cirkel GA, Hamberg P, Sleijfer S, Loosveld OJ, Dercksen MW, Los M, et al. Alternating treatment with pazopanib and everolimus vs continuous pazopanib to delay disease progression in patients with metastatic clear cell renal cell cancer: the ROPETAR randomized clinical trial. JAMA Oncology 2017;3(4):501-8. - PubMed
Climent 2020 {published data only}
-
- Climent Duran MA, Basterretxea L, Sanchez Escribano R, Llabres E, Heras Lopez L, Zambrana F, et al. Pilot study of cabozantinib efficacy, safety and tolerability in metastatic renal carcinoma in aged fragile patients. Annals of Oncology 2020;31 Suppl 4:S608.
Cole 2003 {published data only}
-
- Cole BF, McDermott D, Parker R, Youmans A, Connolly C, Ernstoff M, et al. The impact of treatmen with high-dose interleukin-2 (HD IL-2) or subcutaneous (SC) IL-2/interferon alfa-2b (IFN) on quality of life (QoL) in patients with metastatic renal cell carcinoma (mRCC). Proceedings of The American Society of Clinical Oncology 2003.
Collinson 2012 {published data only}
-
- Collinson FJ, Gregory WM, McCabe C, Howard H, Lowe C, Potrata D, et al. The STAR trial protocol: a randomised multi-stage phase II/III study of sunitinib comparing temporary cessation with allowing continuation, at the time of maximal radiological response, in the first-line treatment of locally advanced/metastatic renal cancer. BMC Cancer 2012;12:598. - PMC - PubMed
Collinson 2018 {published data only}
-
- Collinson F, Brown S, Buckley H, Ainsworth G, Howard H, Poad H, et al. PRISM: a randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. Annals of Oncology 2018;29 Suppl 8:viii331. - PMC - PubMed
Colomba 2021 {published data only}
-
- Colomba E, Flippot R, Dalban C, Negrier S, Chevreau C, Gravis G, et al. Association of statins and nivolumab activity in patients with metastatic renal cell carcinoma (mRCC): results from the phase II nivoren-GETUG AFU 26 trial. Journal of Clinical Oncology. Conference 2021;39(6 Suppl):359.
Conter 2013 {published data only}
-
- Conter HJ, Wood CG, Matin SF, Tamboli P, Millikan RE, Jonasch E, et al. Ten-year follow-up of patients (pts) with metastatic renal cell carcinoma (mRCC) treated with interferon alfa-2b (IFN) as first-line therapy: results from a randomized trial. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):365.
Demirci 1999 {published data only}
-
- Demirci D, Tatlisen A, Ekmekcioglu O, Orskiran G, Gulmez I. Comparison of vinblastine and interferon alpha with 5-flourouracil treatment in metastatic renal cell carcinoma: a preliminary report. Erciyes Tip Dergisi 1999;21(2):111-6.
de Mulder 1991 {published data only}
-
- Mulder PH, Debruyne FM, Oosterom A, Bouffioux C, Vermeylen K, Sylvester R. EORTC randomized phase II study of recombinant interferon alpha and recombinant interferon alpha and gamma in advanced renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 10. 1991:166, Abstract 528.
Dexeus 1988 {published data only}
-
- Dexeus F, Logothetis C, Chong C, Sella A, Finn L. Phase III study in metastatic renal cell carcinoma comparing combination chemotherapy versus interferon alternating with combination chemotherapy. In: Proceedings of The American Society of Clinical Oncology. Vol. 7. 1988:131, Abstract 507.
Dexeus 1989 {published data only}
-
- Dexeus FH, Logothetis CJ, Sella A, Finn L. Interferon alternating with chemotherapy for patients with metastatic renal cell carcinoma. American Journal of Clinical Oncology 1989;12(4):350-4. - PubMed
DRKS00010309 2016 {published data only}
-
- DRKS00010309. A phase 2 randomized, double-blind, crossover, controlled, multi-center subject preference study of tivozanib hydrochloride versus sunitinib in the treatment of subjects with metastatic renal cell carcinoma. drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00... 2016.
Dubois 1997 {published data only}
-
- Dubois JS, Trehu EG, Mier JW, Shapiro L, Epstein M, Klempner M, et al. Randomized placebo-controlled clinical trial of high-dose interleukin-2 in combination with a soluble p75 tumor necrosis factor receptor immunoglobulin G chimera in patients with advanced melanoma and renal cell carcinoma. Journal of Clinical Oncology 1997;15(3):1052-62. - PubMed
Eisen 2019 {published data only}
-
- Eisen TQ, Frangou E, Smith B, Ritchie A, Kaplan RS, Oza B, et al. Primary efficacy analysis results from the SORCE trial (RE05): adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: an international, randomised double-blind phase III trial led by the MRC CTU at UCL. Annals of Oncology 2019;30:v891-2.
Elhilali 2000 {published data only}
-
- Elhilali MM, Gleave M, Fradet Y, Davis I, Venner P, Saad F, et al. Placebo-associated remissions in a multicentre, randomized, double-blind trial of interferon gamma-1b for the treatment of metastatic renal cell carcinoma. BJU International 2000;86(6):613-8. - PubMed
Epaillard 2020 {published data only}
-
- Epaillard N, Simonaggio A, Elaidi R, Azzouz F, Brachychenko E, Thibault C, et al. BIONIKK: a phase 2 biomarker driven trial with nivolumab and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naïve metastatic kidney cancer. Bulletin du Cancer 2020;107(5):eS22-7. - PubMed
Escudier 2005 {published data only}
-
- Escudier B, Szczylik C, Eisen T, Stadler WM, Schwartz B, Shan M, et al. Randomized phase III trial of the raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). In: Annual meeting proceedings of The American Society of Clinical Oncology. Vol. 23. 2005:380.
Euctr2006‐002851‐33‐AT {published data only}
-
- Schmidinger M. Sorafenib and bevacizumab as first- line treatment in patients with advanced renal cell carcinoma. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-002851-33-AT 2006.
Euctr 2006‐003429‐95‐ES {published data only}
-
- EUCTR2006-003429-95-ES. Treatment for renal cancer. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-003429-95-ES 2012.
Euctr2006‐005751‐16‐NL {published data only}
-
- EUCTR2006-005751-16-NL. An open label, dose escalation safety and tolerability trial of the combination of s.c. recombinant human IL-21 (rIL-21) and sunitinib (phase 1) followed by an open label stratified randomized 2-arm trial of rIL-21 plus sunitinib versus sunitinib alone (phase 2a) in subjects with stage IV renal cell carcinoma. Trial phase: 1/2a. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-005751-16-NL 2007.
Euctr2007‐002556‐41‐AT {published data only}
-
- EUCTR2007-002556-41-AT. TWIST- randomized prospective phase II study of temsirolimus with or without low-dose interferon alpha in metastatic non-clear renal cell carcinoma- GOIRC STUDY 02/2008 - TWIST. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007%E2%80%90002556%E2%80%9... 2008.
EUCTR2008‐002667‐13‐DE 2008 {published data only}
-
- EUCTR2008-002667-13-DE. A single-center controlled pilot study to investigate pre- and perioperative therapy with Sorafenib (Nexavar®) in patients who are candidates for a curative surgery of renal cell cancer (PREST = preoperative Sorafenib Therapy in RCC) - PREST. https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-002667-13/ 2008.
Euctr2012‐001730‐33‐ES {published data only}
-
- EUCTR2012-001730-33-ES. A phase 2 randomized, double-blind, crossover, controlled, multi-center, subject preference study of tivozanib hydrochloride versus sunitinib in the treatment of subjects with metastatic renal cell carcinoma. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-001730-33-ES 2012.
Euctr2015‐002133‐22‐FR {published data only}
-
- EUCTR2015-002133-22-FR. Study of the drug substances MLN0128 and MLN0128+MLN1117 compared with everolimus in the treatment of patients with metastatic clear cell renal carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-002133-22-FR 2017.
Euctr2018‐001495‐38‐FR {published data only}
-
- EUCTR2018-001495-38-FR. A randomized, phase 3, double-blind, placebo-controlled study of Pazopanib with or without abexinostat in patients with locally advanced or metastatic renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001495-38-FR 2018.
Feldman 2020 {published data only}
Feldman 2020a {published data only}
Figlin 1998 {published data only}
-
- Figlin RA, Thompson JA, Roudet C, Lange P, Belldegrun A. Multi-center randomized placebo controlled phase II/III trial of CD8(+) tumor infiltrating lymphocyte therapy (CD8(+) TIL)/recombinant interleukin-2 (IL-2) in metastatic renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 17. 1998:318a, Abstract 1225.
Figlin 1999 {published data only}
-
- Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. Journal of Clinical Oncology 1999;17(8):2521-9. - PubMed
Figlin 2014 {published data only}
-
- Figlin RA, Wood CG. Patient identification and eligibility insights in the synchronous mRCC population: an update from the ongoing ADAPT* phase 3 study experience. In: Journal of Clinical Oncology (Conference). Vol. 33. 2014.
Figlin 2014a {published data only}
-
- Figlin RA, Wood CG. Enrollment insights in the synchronous mRCC population: an update from the ongoing ADAPT* phase 3 study experience. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 32. 2014.
Figlin 2017 {published data only}
-
- Figlin R, Nicolette C, Tannir N, Tykodi SS, Chen D, Master V, et al. Interim analysis of the phase 3 ADAPT trial evaluating rocapuldencel-T (AGS-003), an individualized immunotherapy for the treatment of newly-diagnosed patients with metastatic renal cell carcinoma (mRCC). Annals of Oncology 2017;28 Suppl 5:v404.
Figlin 2018 {published data only}
-
- Figlin RA, Wood CG, Gamble AH, Plachco A, Norris MS, Horvatinovich JM, et al. Interim data analysis of the ADAPT trial using the modified intent to treat (mITT) population re-evaluates Rocapuldencel-T for clinical benefit over standard of care. Journal of Clinical Oncology: ASCO annual meeting proceedings 2018;36(15 Suppl 1):4557.
Figlin 2020 {published data only}
-
- Figlin RA, Tannir NM, Uzzo RG, Tykodi SS, Chen DY, Master V, et al. Results of the ADAPT phase 3 study of rocapuldencel-t in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clinical Cancer Research 2020;26(10):2327-35. - PubMed
Flaherty 2015 {published data only}
-
- Flaherty KT, Manola JB, Pins M, McDermott DF, Atkins MB, Dutcher JJ, et al. BEST: a randomized phase II study of vascular endothelial growth factor, RAF kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma - a trial of the ECOG-ACRIN cancer research group (E2804). Journal of Clinical Oncology 2015;33(21):2384-91. - PMC - PubMed
Foon 1988 {published data only}
-
- Foon K, Doroshow J, Bonnem E, Fefer A, Graham S, Grosh B, et al. A prospective randomized trial of alpha(2B)-interferon/gamma- interferon or the combination of advanced metastatic renal cell carcinoma. Journal of Biological Response Modifiers 1988;7(6):540-5. - PubMed
Fossa 1989 {published data only}
-
- Fossa SD, Raabe N, Moe B. Recombinant interferon-alpha with or without vinblastine in metastatic renal carcinoma. Results of a randomised phase II study. British Journal of Urology 1989;64(5):468-71. - PubMed
Fossa 1992 {published data only}
-
- Fossa SD, Martinelli G, Otto U, Schneider G, Wander H, Oberling F, et al. Recombinant interferon alfa-2a with or without vinblastine in metastatic renal cell carcinoma: results of a European multi-center phase III study. Annals of Oncology 1992;3(4):301-5. - PubMed
Gao 2017 {published data only}
-
- Gao J, Karam JA, Wood CG, Matin S, Ahrar K, Jonasch E, et al. Clinical activity, immune and molecular correlates of nivolumab vs. nivolumab plus bevacizumab vs nivolumab plus ipilimumab in metastatic renal cell carcinoma. Cancer Research 2017;77(13 Suppl):CT083.
Gao 2019 {published data only}
-
- Gao J, Karam JA, Tannir NM, Campbell MT, Tidwell RS, Ahrar K, et al. A pilot randomized study evaluating nivolumab (nivo) or nivo + bevacizumab (bev) or nivo + ipilimumab (ipi) in patients with metastatic renal cell carcinoma (MRCC) eligible for cytoreductive nephrectomy, metastasectomy or post-treatment biopsy (Bx). Journal of Clinical Oncology 2019;37(15):4501.
Gedye 2021 {published data only}
-
- Gedye C, Joshi AJ, Zhang AY, Martin AJ, Joshua AM, Harris CA, et al. Denosumab and pembrolizumab in clear cell renal carcinoma (KEYPAD): a phase II trial (ANZUP1601). In: Journal of Clinical Oncology (Conference). Vol. 39. 2021.
Ghiorghiu 2018 {published data only}
-
- Ghiorghiu D, Jakacki R, Haddad V, Kohlmann A, Frigault MM, Ottesen L, et al. Savolitinib versus sunitinib in patients with MET-driven, unresectable and locally advanced or metastatic papillary renal cell carcinoma: SAVOIR, a randomised, phase III trial. Kidney Cancer 2018;2 Suppl 1:S33.
Gleave 1997 {published data only}
-
- Gleave M, Elhilai M, Fradet Y, Davis I, Venner P, Saad F, et al. A multicenter randomized, double blind trial of actimmune(r) interferon gamma-1b injection versus placebo for the treatment of metastatic renal cell carcinoma. In: Proceedings of the Annual Meeting of the American Society for Clinical Oncology. 1997.
Gleave 1997a {published data only}
-
- Gleave M, Elhilali M, Fradet Y. A phase III randomized double-blind placebo-controlled multicenter trial comparing interferon gamma to placebo in patients with metastatic renal cell carcinoma. Journal of Urology 1997;157:A1274.
Gleave 1998 {published data only}
-
- Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian urologic oncology group. New England Journal of Medicine 1998;338(18):1265-71. - PubMed
Gore 2008 {published data only}
-
- Gore ME. Interferon-a (IFN), interleukin-2 (IL2) and 5-fluorouracil (5FU) vs IFN alone in patients with metastatic renal cell carcinoma (mRCC): results of the randomised MRC/EORTC RE04 trial. Journal of Clinical Oncology 2008;26(15 Pt 1):259.
Gore 2010 {published data only}
-
- Gore ME, Griffin CL, Hancock B, Patel PM, Pyle L, Aitchison M, et al. Interferon alfa-2a versus combination therapy with interferon alfa-2a, interleukin-2, and fluorouracil in patients with untreated metastatic renal cell carcinoma (MRC RE04/EORTC GU 30012): an open-label randomised trial. Lancet 2010;375(9715):641-8. - PMC - PubMed
Gruenwald 2020 {published data only}
-
- Gruenwald V, Ivanyi P, Jacobasch L, Zahn MO, Hubner A, Von Weikersthal LF, et al. A phase III study testing the role of proactive coaching on patient reported outcome in advanced or metastatic renal cell carcinoma treated with sunitinib or a combination of axitinib + checkpoint inhibitor (CPI) in first line therapy (PREPARE). Oncology Research and Treatment 2020;43:224.
Haas 2016 {published data only}
Hainsworth 2015 {published data only}
-
- Hainsworth JD, Mace JR, Reeves JA, Crane EJ, Hamid O, Stille JR, et al. Randomized phase II study of sunitinib + CXCR4 inhibitor LY2510924 versus sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma. Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4547.
Hainsworth 2016 {published data only}
-
- Hainsworth JD, Reeves JA, Mace JR, Crane EJ, Hamid O, Stille JR, et al. A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Targeted Oncology 2016;11(5):643-53. - PubMed
Han 2002 {published data only}
-
- Han KR, Pantuck AJ, Belldegrun AS. A randomized phase III trial of high dose interleukin-2 versus subcutaneous interleukin-2/interferon. Current Urology Reports 2002;3(1):11-2. - PubMed
Harima 1990 {published data only}
-
- Harima M, Yasumoto R, Asakawa M, Horii A, Nishio S, Sakamoto W, et al. Comparison of efficacy between interferon-alpha (IFN) plus fluoropyrimidine (FP) and IFN alone in advanced renal cell cancer (RCC): preliminary results. Journal of Cancer Research and Clinical Oncology 1990;116:533.
Henriksson 1998 {published data only}
Hutson 2006 {published data only}
-
- Hutson TE, Bukowski RM. A phase II study of GW786034 using a randomized discontinuation design in subjects with locally recurrent or metastatic clear-cell renal cell carcinoma. Clinical Genitourinary Cancer 2006;4(4):296-8. - PubMed
Hutson 2021 {published data only}
-
- Hutson TE, Carthon BC, Yorio J, Babu S, McKean HA, Percent IJ, et al. Safety and efficacy outcomes with nivolumab plus ipilimumab in patients with advanced renal cell carcinoma and low Karnofsky performance status: results from the CheckMate 920 trial. In: Journal of Clinical Oncology. Conference. Vol. 39. 2021.
ISRCTN95351638 {published data only}
-
- Collinson F, Brown S, Buckley H, Ainsworth G, Howard H, Poad H, et al. PRISM: a randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. Annals of Oncology 2018;29 Suppl 8:viii331. - PMC - PubMed
-
- EUCTR2017-001476-33-GB. A randomised phase II trial comparing two different ways of combining nivolumab and ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-001476-33-GB 2018.
Jager 2005 {published data only}
-
- Jager E, Heinzer H, Grimm MO, Krause S, Scheuring U, Siebels M. Randomized phase III trial of the multiple kinase inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). In: Onkologie. Vol. 28 Suppl 3. 2005:1–275.
Jayson 1998 {published data only}
Jeon 1999 {published data only}
-
- Jeon SH, Chang SG. The effect of immunotherapy based on interferon - alpha in advanced renal cell carcinoma. Journal of the Korean Cancer Association 1999;31(5):986-94.
JPRN‐JapicCTI‐122014 {published data only}
-
- JPRN-JapicCTI-122014. A Phase 3 Study of ONO-4538/BMS-936558. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-122014 2012.
JPRN‐jRCTs031180024 {published data only}
-
- JPRN-jRCTs031180024. NIVOIGERCC. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs031180024 2018.
JPRN‐UMIN000001995 {published data only}
-
- JPRN-UMIN000001995. Randomized parallel group phase II clinical study of sorafenib/interferon combination therapy and sunitinib monotherapy for advanced renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000001995 2009.
Kinouchi 2004 {published data only}
Kinouchi 2006 {published data only}
-
- Kinouchi T, Sakamoto J, Tsukamoto T, Akaza H, Kubota Y, Ozono S, et al. Prospective randomized trial of natural interferon-alpha versus natural interferon-alpha plus cimetidine in advanced renal cell carcinoma with pulmonary metastasis. Journal of Cancer Research and Clinical Oncology 2006;132(8):499-504. - PMC - PubMed
Larkin 2019 {published data only}
-
- Larkin JM, Tykodi SS, Donskov F, Lee JL, Szczylik C, Malik J, et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): updated follow-up for KEYNOTE-427 cohort A. Annals of Oncology 2019;30 Suppl 5:v381-2.
Law 1995 {published data only}
-
- Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O'Connell M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine- activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995;76(5):824-32. - PubMed
Lee 2020 {published data only}
-
- Lee JL, Ziobro M, Suarez C, Langiewitz P, Matveev VB, Wiechno P, et al. First-line pembrolizumab (pembro) monotherapy in advanced non-clear cell renal cell carcinoma (nccRCC): updated follow-up for KEYNOTE-427 cohort B. Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15):5034.
Lee 2021 {published data only}
-
- Lee CH, Voss MH, Carlo MI, Chen YB, Reznik E, Knezevic A, et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: results of a phase 2 trial. Journal of Clinical Oncology: ASCO annual meeting proceedings 2021;39(15 Suppl):4509.
Lindskog 2020 {published data only}
-
- Lindskog M, Laurell A, Kjellman A, Melichar B, Niezabitowski J, Maroto P, et al. A randomized phase II study with ilixadencel, a cell-based immune primer, plus sunitinib versus sunitinib alone in synchronous metastatic renal cell carcinoma. Journal of Clinical Oncology 2020;38(5):11.
Lissoni 1993 {published data only}
-
- Lissoni P, Barni S, Ardizzoia A, Andres M, Scardino E, Cardellini P, et al. A randomized study of low-dose interleukin-2 subcutaneous immunotherapy versus interleukin-2 plus interferon-alpha as first line therapy for metastatic renal cell carcinoma. Tumori 1993;79(6):397-400. - PubMed
Liu 2012 {published data only}
-
- Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clinical Cancer Research 2012;18(6):1751-9. - PubMed
Lummen 1996 {published data only}
-
- Lummen G, Goepel M, Mollhoff S, Hinke A, Otto T, Rubben H. Phase II study of interferon-gamma versus interleukin-2 and interferon-alpha2b in metastatic renal cell carcinoma. Journal of Urology 1996;155(2):455-8. - PubMed
Madhusudan 2004 {published data only}
-
- Madhusudan S, Protheroe A, Vasey P, Patel P, Selby P, Altman D, et al. A randomized phase II study of interferon alpha alone or in combination with thalidomide in metastatic renal cancer. Journal of Clinical Oncology 2004;22(14 Suppl):4742.
McDermott 2001 {published data only}
-
- McDermott D, Flaherty L, Clark J, Weiss G, Logan T, Gordon M, et al. A randomized phase III trial of high-dose interleukin-2 (HD IL2) versus subcutaneous (SC) Il2/interferon (IFN) in patients with metastatic renal cell carcinoma (RCC). In: Proceedings of The American Society of Clinical Oncology. Vol. 20 Pt 1. 2001:172a, Abstract 685. - PubMed
McDermott 2005 {published data only}
-
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2005;23(1):133-41. - PubMed
McDermott 2013 {published data only}
-
- McDermott DF, Manola J, Pins M, Flaherty KT, Atkins MB, Dutcher JP, et al. The BEST trial (E2804): a randomized phase II study of VEGF, RAF kinase, and mTOR combination targeted therapy (CTT) with bevacizumab (bev), sorafenib (sor), and temsirolimus (tem) in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):345. - PMC - PubMed
McDermott 2020 {published data only}
-
- McDermott DF, Lee JL, Bjarnason GA, Larkin JM, Gafanov R, Kochenderfer MD, et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): updated follow-up for KEYNOTE-427 cohort A. Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15):5069.
Mickisch 2001 {published data only}
-
- Mickisch GH, Garin A, Poppel H, Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358(9286):966-70. - PubMed
Minasian 1993 {published data only}
-
- Minasian LM, Motzer RJ, Gluck L, Mazumdar M, Vlamis V, Krown SE. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. Journal of Clinical Oncology 1993;11(7):1368-75. - PubMed
Molina 2009 {published data only}
-
- Molina AM, Ginsberg M, Sweeney S, Myrie J, Motzer R J. Efficacy and safety of everolimus treatment in metastatic renal cell carcinoma. American Journal of Hematology/Oncology 2009;8(4):166-169.
Motzer 2001 {published data only}
-
- Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha2a for patients with advanced renal cell carcinoma. Journal of Interferon and Cytokine Research 2001;21(4):257-63. - PubMed
Mulders 2012 {published data only}
-
- Mulders P, Hawkins R, Nathan P, Jong I, Osanto S, Porfiri E, et al. Cediranib monotherapy in patients with advanced renal cell carcinoma: results of a randomised phase II study. European Journal of Cancer 2012;48(4):527-37. - PubMed
Naglieri 1998 {published data only}
-
- Naglieri E, Gebbia V, Durini E, Lelli G, Abbate I, Selvaggi FP, et al. Standard interleukin-2 (IL-2) and interferon-alpha immunotherapy versus an IL-2 and 4-epirubicin immuno-chemotherapeutic association in metastatic renal cell carcinoma. Anticancer Research 1998;18(3B):2021-6. - PubMed
NCT00002737 {published data only}
-
- NCT00002737. Interferon alfa with or without isotretinoin in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00002737 2004.
NCT00005966 {published data only}
-
- NCT00005966. Interferon alfa-2b with or without thalidomide in treating patients with metastatic or unresectable kidney cancer. clinicaltrials.gov/show/NCT00005966 2003.
NCT00019539 {published data only}
-
- NCT00019539. Monoclonal antibody therapy in treating patients with advanced kidney cancer. clinicaltrials.gov/show/NCT00019539 2009.
NCT00027664 {published data only}
-
- NCT00027664. Interferon alfa with or without thalidomide in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00027664 2003.
NCT00053820 {published data only}
-
- NCT00053820. Interferon alfa with or without interleukin-2 and fluorouracil in treating patients with advanced metastatic kidney cancer. clinicaltrials.gov/show/NCT00053820 2003.
NCT00073307 {published data only}
-
- Eisen T, Bukowski RM, Staehler M, Szczylik C, Oudard S, Stadler WM, et al. Randomized phase III trial of sorafenib in advanced renal cell carcinoma (RCC): impact of crossover on survival. Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18S):4524.
-
- Negrier S, Jager E, Porta C, McDermott D, Moore M, Bellmunt J, et al. Efficacy and safety of sorafenib in patients with advanced renal cell carcinoma with and without prior cytokine therapy, a subanalysis of TARGET. Medical Oncology 2010;27(3):899-906. - PubMed
NCT00100906 {published data only}
-
- NCT00100906. Sequential ATRA then IL-2 for modulation of dendritic cells and treatment of metastatic renal cell cancer. clinicaltrials.gov/show/NCT00100906 2005.
NCT00378703 {published data only}
-
- NCT00378703. Bevacizumab, sorafenib tosylate, and temsirolimus in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00378703 2006.
NCT00416871 {published data only}
-
- NCT00416871. Interleukin-2 and interferon in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00416871 2006.
NCT00467025 {published data only}
-
- Inc Amgen. A randomized, double blinded, multi-center study 2 study to estimate the efficacy and evaluate the safety and tolerability of sorafenib in combination with AMG 386 or placebo in subjects with metas. https://clinicaltrials.gov/ct2/show/NCT00467025.
NCT00491738 {published data only}
-
- NCT00491738. A study evaluating the efficacy and safety of sunitinib with or without bevacizumab in first-line patients with metastatic renal cell cancer (SABRE-R). clinicaltrials.gov/show/NCT00491738 2007.
NCT00709995 {published data only}
-
- NCT00709995. A study for participants with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT00709995 2008.
NCT00835978 {published data only}
-
- Rini BI, Gruenwald V, Fishman MN, Melichar B, Ueda T, Karlov P, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. Clinical Advances in Hematology and Oncology 2012;10(9):6-8.
-
- Rini BI, Melichar B, Fishman MN, Oya M, Pithavala YK, Chen Y et al. Axitinib dose titration: analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Annals of Oncology 2015;26(7):1372-7. - PubMed
-
- Rini BI, Tomita Y, Melichar B, Ueda T, Gruenwald V, Fishman MN, et al. Overall survival analysis from a randomized phase II study of axitinib with or without dose titration in first-line metastatic renal cell carcinoma. Clinical Genitourinary Cancer 2016;14(6):499-503. - PubMed
NCT00873236 {published data only}
-
- EUCTR2008-006414-19-GB. Dynamic contrast enhanced MRI (DCE-MRI) assessment of the vascular changes induced with bevacizumab alone and in combination with interferon-a in patients with advanced renal cell carcinoma. - DCE-MRI changes in tumour vaculature with Bevacizumab and Interferon in advanced RCC. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-006414-19-GB 2009.
NCT01164228 {published data only}
-
- NCT01164228. Sunitinib malate with or without gemcitabine hydrochloride in treating patients with advanced kidney cancer that cannot be removed by surgery. clinicaltrials.gov/show/NCT01164228 2010.
NCT01223027 {published data only}
-
- NCT01223027. Study of dovitinib versus sorafenib in patients with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT01223027 2010.
NCT01408004 {published data only}
-
- NCT01408004. Rotating pazopanib and everolimus to avoid resistance. clinicaltrials.gov/show/NCT01408004 2011.
NCT01444807 {published data only}
-
- NCT01444807. Evaluate the efficacy of sorafenib in renal cell carcinoma patients after a radical resection of the metastases. clinicaltrials.gov/show/NCT01444807 2011.
NCT01616186 {published data only}
-
- NCT01616186. Everolimus/sorafenib or sunitinib in patients with metastatic renal cell carcinoma (RCC). clinicaltrials.gov/show/NCT01616186 2012.
NCT01664182 {published data only}
-
- NCT01664182. Trebananib with or without bevacizumab, pazopanib hydrochloride, sorafenib tosylate, or sunitinib malate in treating patients with advanced kidney cancer. clinicaltrials.gov/show/NCT01664182 2012.
NCT01673386 {published data only}
-
- NCT01673386. A subject treatment preference study of tivozanib hydrochloride versus sunitinib in subjects with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT01673386 2012.
NCT01727089 {published data only}
-
- NCT01727089. Bevacizumab with or without TRC105 in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT01727089 2012.
NCT01727336 {published data only}
-
- NCT01727336. Study of dalantercept and axitinib in patients with advanced renal cell carcinoma. clinicaltrials.gov/show/NCT01727336 2012.
NCT01793636 {published data only}
-
- NCT01793636. A study comparing AZD2014 vs everolimus in patients with metastatic renal cancer. clinicaltrials.gov/show/NCT01793636 2013.
NCT02014636 {published data only}
-
- NCT02014636. Safety and efficacy study of pazopanib and MK 3475 in advanced renal cell carcinoma (RCC; KEYNOTE-018). clinicaltrials.gov/show/NCT02014636 2013.
NCT02127710 {published data only}
-
- EUCTR2014-001858-41-ES. This is a study designed to evaluate the effectiveness and safety of AZD6094 in patients with papillary renal cell cancer. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-001858-41-ES 2015.
NCT02667886 {published data only}
-
- NCT02667886. Trial of X4P-001 in patients with advanced renal cell carcinoma. clinicaltrials.gov/show/NCT02667886 2016.
NCT02724020 {published data only}
-
- NCT02724020. MLN0128 and MLN0128 + MLN1117 compared with everolimus in the treatment of adults with advanced or metastatic clear-cell renal cell carcinoma. clinicaltrials.gov/show/NCT02724020 2016.
NCT02960906 {published data only}
-
- Epaillard N, Simonaggio A, Elaidi R, Azzouz F, Braychenko E, Thibault C et al. A BIOmarker driven trial with nivolumab and ipilimumab or VEGFR tKi in naive metastatic kidney cancer (BIONIKK). clinicaltrials.gov/ct2/show/NCT02960906. - PubMed
NCT03035630 {published data only}
-
- NCT03035630. Sunitinib followed by avelumab or the reverse for metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT03035630 2017.
NCT03092856 {published data only}
-
- NCT03092856. Axitinib with or without anti-OX40 antibody PF-04518600 in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT03092856 2017.
NCT03095040 {published data only}
-
- NCT03095040. CM082 combined with everolimus in chinese patients with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT03095040 2017.
NCT03173560 {published data only}
-
- NCT03173560. Trial to assess safety and efficacy of lenvatinib (18 mg vs. 14 mg) in combination with everolimus in participants with renal cell carcinoma. clinicaltrials.gov/ct2/show/NCT03173560 2017.
NCT03501381 {published data only}
-
- NCT03501381. High dose IL 2 and entinostat in RCC. clinicaltrials.gov/show/NCT03501381 2018.
NCT03595124 {published data only}
-
- NCT03595124. Axitinib and nivolumab in treating participants with unresectable or metastatic TFE/translocation renal cell carcinoma. clinicaltrials.gov/show/NCT03595124 2018.
NCT03829111 {published data only}
-
- NCT03829111. CBM588, nivolumab, and ipilimumab in treating patients with stage IV or advanced kidney cancer. clinicaltrials.gov/show/NCT03829111 2019.
NCT04195750 {published data only}
-
- NCT04195750. A study of MK-6482 versus everolimus in participants with advanced renal cell carcinoma (MK-6482-005). clinicaltrials.gov/show/NCT04195750 2019.
NCT04300140 {published data only}
-
- NCT04300140. Efficacy and safety study of AVB-S6-500 in patients with clear cell renal cell carcinoma. clinicaltrials.gov/show/NCT04300140 2020.
Negrier 1996 {published data only}
-
- Negrier S, Escudier B, Lasset C, Savary J, Douillard JY, Chevreau C, et al. The FNCLCC Crecy trial: interleukin 2 (IL2) + interferon (IFN) is the optimal treatment to induce responses in metastatic renal cell carcinoma (mRCC). In: Proceedings of The American Society of Clinical Oncology. Vol. 15. 1996:248, Abstract 629.
Negrier 1997 {published data only}
-
- Negrier S, Escudier B, Douillard JY, Lesimple T, Rossi JF, Viens P, et al. Randomized study of interleukin-2 and interferon with or without 5-FU (FUCY study) in metastatic renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 16. 1997:326a, Abstract 1161.
Negrier 1998 {published data only}
-
- Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. New England Journal of Medicine 1998;338(18):1272-8. - PubMed
Negrier 2000 {published data only}
-
- Negrier S, Caty A, Lesimple T, Douillard JY, Escudier B, Rossi JF, et al. Treatment of patients with metastatic renal carcinoma with a combination of subcutaneous interleukin-2 and interferon alfa with or without fluorouracil. Groupe Francais d'Immunotherapie, Federation Nationale des Centres de Lutte Contre le Cancer. Journal of Clinical Oncology 2000;18(24):4009-15. - PubMed
Negrier 2006 {published data only}
-
- Negrier S, Perol D, Ravaud A, Bay JO, Oudard S, Fargeot P, et al. Is intravenous (iv) IL2 superior to subcutaneous (sc) IL2 in good prognosis patients (pts) with metastatic renal cell carcinoma (mRCC) receiving a combination of IL2 and alpha interferon (IFN)? Results of the prospective randomized PERCY duo trial. Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18 Suppl):4536.
Negrier 2007 {published data only}
-
- Negrier S, Perol D, Ravaud A, Chevreau C, Bay JO, Delva R, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 2007;110(11):2468-77. - PubMed
Negrier 2008 {published data only}
-
- Negrier S, Perol D, Ravaud A, Bay JO, Oudard S, Chabaud S, et al. Randomized study of intravenous versus subcutaneous interleukin-2, and IFNalpha in patients with good prognosis metastatic renal cancer. Clinical Cancer Research 2008;14(18):5907-12. - PubMed
Nosov 2010 {published data only}
-
- Nosov DA, Bhargava P, Esteves B, Al-Adhami M, Lipatov O, Lyulko AA, et al. Phase 2 randomized discontinuation trial (RDT) of tivozanib in patients with renal cell carcinoma (RCC): results in patients randomized to tivozanib vs. placebo. Annals of Oncology 2010;8:viii271.
Nosov 2012 {published data only}
-
- Nosov DA, Bhargava P, Esteves B, Strahs AL, Lipatov ON, Lyulko AA, et al. Final analysis of the phase 2 randomized discontinuation trial of tivozanib (AV-951) versus placebo in patients with renal cell carcinoma. Journal of Clinical Oncology 2012;29(15 Suppl):4550. - PubMed
Pal 2015 {published data only}
Pal 2021a {published data only}
-
- Pal SK, Puente J, Heng DY, Glen H, Koralewski P, Stroyakovskiy D, et al. Phase II trial of lenvatinib (LEN) at two starting doses + everolimus (EVE) in patients (pts) with renal cell carcinoma (RCC): results by independent imaging review (IIR) and prior immune checkpoint inhibition (ICI). Journal of Clinical Oncology 2021;39(6 Suppl):307.
Passalacqua 2010 {published data only}
Plimack 2015 {published data only}
-
- Plimack ER, Hammers HJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, et al. Updated survival results from a randomized, doseranging phase II study of nivolumab (NIVO) in metastatic renal cell carcinoma (MRCC). Journal of Clinical Oncology 2015;33(15 Suppl):4553.
Pyrhonen 1995 {published data only}
-
- Pyrhonen S, Salminen E, Lehtonen T, Nurmi M, Tammela T, Juusela H, et al. Recombinant interferon alfa-2a (IFN) with vinblastine vs vinblastine alone in advanced renal cell carcinoma (RCC). A phase III study. In: Proceedings of The American Society of Clinical Oncology. Vol. 14. 1995:253, Abstract 686.
Pyrhonen 1996 {published data only}
-
- Pyrhonen S, Salminen E, Lehtonen T, Nurmi M, Tammela T, Juusela H, et al. Recombinant interferon alfa-2a with vinblastine vs vinblastine alone in advanced renal cell carcinoma: a phase III study. In: Proceedings of The American Society of Clinical Oncology. Vol. 15. 1996:244, Abstract 614.
Pyrhonen 1999 {published data only}
-
- Pyrhonen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. Journal of Clinical Oncology 1999;17(9):2859-67. - PubMed
Ravaud 2006 {published data only}
-
- Ravaud A, Gardner J, Hawkins R, Maase H, Zantl N, Harper P, et al. Efficacy of lapatinib in patients with high tumor EGFR expression: results of a phase III trial in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2006 2006;24(18 Suppl):4502.
Ravaud 2016 {published data only}
-
- Ravaud A, Motzer RJ, Pandha HS, Staehler M, George D, Pantuck AJ, et al. PR phase III trial of sunitinib (SU) vs placebo (PBO) as adjuvant treatment for high-risk renal cell carcinoma (RCC) after nephrectomy (S-TRAC). Annals of Oncology 2016;27(6):1-36.
Rexer 2017 {published data only}
-
- Rexer H, Steiner T, Gruenwald V. First-line therapy in advanced renal cell carcinoma: a randomized phase II study to examine early switch of tyrosine kinase inhibitors to nivolumab compared to continued tyrosine kinase inhibitor therapy in patients with advanced or metastatic renal cell carcinoma and stable disease after three months of treatment (NIVOSWITCH)-AN 38/15 of the AUO.. Urologe 2017;56(4):509-11. - PubMed
Richards 1977 {published data only}
-
- Richards F, Muss HB, White DR, Cooper MR, Spurr CL. CCNU, bleomycin, and methylprednisolone with or without adriamycin in renal cel carcinoma: a randomized trial. Cancer Treatment Reports 1977;61(8):1591-3. - PubMed
Rini 2011 {published data only}
-
- Rini BI, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, et al. AMG 386 in combination with sorafenib in patients (pts) with metastatic renal cell cancer (mRCC): a randomized, double-blind, placebo-controlled, phase II study. Journal of Clinical Oncology. Conference 2011;29(7 Suppl 1):309. - PMC - PubMed
Rini 2012 {published data only}
Rodriguez‐Vida 2020 {published data only}
-
- Rodriguez-Vida A, Bamias A, Esteban E, Saez MI, Lopez-Brea M, Castellano D, et al. Randomised phase II study comparing alternating cycles of sunitinib and everolimus vs standard sequential administration in first-line metastatic renal carcinoma (SUNRISES study). BJU international 2020;126(5):559-67. - PubMed
Rpcec 2017 {published data only}
-
- RPCEC00000229. HEBERFERON in renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=RPCEC00000229 2017.
Sternberg 2013 {published data only}
-
- Sternberg C, Bracarda S, Carteni G, Lo Re G, Ruggeri EM, Masini C. Sunitinib expanded-access trial in metastatic renal cell carcinoma (mRCC) - final Italian results. European Journal of Cancer 2013;49:S644.
Szarek 2021 {published data only}
-
- Szarek M, Needle MN, Rini BI, Pal SK, McDermott DF, Atkins MB, et al. Q-TWiST analysis of tivozanib versus sorafenib in patients with advanced renal cell carcinoma in the TIVO-3 study. Clinical Genitourinary Cancer 2021;19(5):468.e1-468.e5. - PubMed
Tannir 2016 {published data only}
Taylor 2020 {published data only}
Taylor 2020a {published data only}
Thiam 2010 {published data only}
-
- Thiam R, Cuenod CA, Fournier LS, Lamuraglia M, Medioni J, Barascout B, et al. Determination of a new recist threshold using everolimus treatment in metastatic renal cell carcinoma: evaluation from the RECORD-1 study. Annals of Oncology 2010;8:viii74-5.
Trump 2004 {published data only}
-
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the german cooperative renal carcinoma chemoimmunotherapy group (DGCIN). Journal of Clinical Oncology 2004;22(7):1188-94. - PubMed
Twardowski 2015 {published data only}
-
- Twardowski P, Plets M, Plimack ER, Agarwal N, Tangen CM, Vogelzang NJ, et al. SWOG 1107: parallel (randomized) phase II evaluation of tivantinib (ARQ-197) and tivantinib in combination with erlotinib in patients (pts) with papillary renal cell carcinoma (pRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4523.
Twardowski 2017 {published data only}
Verzoni 2018 {published data only}
-
- Verzoni E, Ratta R, Grassi P, Salvioni R, Stagni S, Montone R, et al. TARIBO trial: targeted therapy with or without nephrectomy in metastatic renal cell carcinoma: liquid biopsy for biomarkers discovery. Tumori 2018;104(5):401-5. - PubMed
Voss 2015 {published data only}
-
- Voss MH, Chen Y, Chaim J, Coskey DT, Woo K, Redzematovic A, et al. A phase II trial of everolimus (E) and bevacizumab (B) in advanced non-clear cell renal cell cancer (ncRCC) to show efficacy in patients (pts) with papillary features. Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4522.
Voss 2019 {published data only}
-
- Voss MH, Azad AA, Hansen AR, Gray JE, Welsh SJ, Achour I, et al. Results from a randomised phase I/II trial evaluating the safety and antitumour activity of anti-PD-1 (MEDI0680)/anti-PD-L1 (durvalumab) vs anti-PD-1 (nivolumab) alone in metastatic clear cell renal cell carcinoma (ccRCC). Annals of Oncology 2019;30:v516.
Witte 1995 {published data only}
-
- Witte RS, Leong T, Emstoff MS, Krigel RL, Oken MM, Harris J, et al. A phase II study of interleukin-2 with and without beta-interferon in the treatment of advanced renal cell carcinoma. Investigational New Drugs 1995;13(3):241-7. - PubMed
Wood 2013 {published data only}
-
- Wood GC, Figlin RA. ADAPT: an ongoing international phase 3 randomized trial of autologous dendritic cell immunotherapy (AGS 003) plus standard treatment in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology 2013;32(4 Suppl):449.
Wright 2020 {published data only}
-
- Wright KM. Final data analysis supports tivozanib as superior treatment for patients with RCC. Oncology 2020;34(7):257. - PubMed
Yang 2002 {published data only}
-
- Yang JC, Haworth L, Steinberg SM, Rosenberg SA, Novotny W. A randomized double-blind placebo-controlled trial of bevacizumab (anti-VEGF antibody) demonstrating a prolongation in time to progression in patients with metastatic renal cancer. Proceedings of The American Society of Clinical Oncology 2002;21 Pt 1:5a, Abstract 15.
Yang 2003 {published data only}
Zhou 2016 {published data only}
-
- Zhou AP, Ma J, Bai Y, Song Y, Li H, Xie X, et al. Anlotinib versus sunitinib as first line treatment for metastatic renal cell carcinoma (mRCC): preliminary results from a randomized phase II clinical trial. Journal of Clinical Oncology. Conference 2016;34(15 Suppl):4565.
References to studies awaiting assessment
Liu 2017 {published data only}
-
- Liu C, Wang X, Mo, J, Yang H, Liu H, Liao H. Efficacy of sunitinib in the treatment of advanced renal cellcarcinoma and its effect on survival time [Chinese]. Anti-Tumor Pharmacy 2017;7(2):195-9.
NCT01217931 {published data only}
-
- Sequential Two-agent Assessment in Renal Cell Carcinoma Therapy: The START Trial. https://www.clinicaltrials.gov/ct2/show/NCT01217931.
NCT01688973 {published data only}
-
- Tivantinib with or without erlotinib hydrochloride in treating patients eith metastatic or locally advanced kidney cancer that cannot be removed by surgery. https://www.clinicaltrials.gov/ct2/show/NCT01688973.
NCT01829841 {published data only}
-
- A Study of famitinib in patients with advanced metastatic renal cell cancer. https://www.clinicaltrials.gov/ct2/show/NCT01829841.
NCT03541902 {published data only}
-
- Cabozantinib or sunitinib malate in treating participants with metastatic variant histology renal cell carcinoma. https://www.clinicaltrials.gov/ct2/show/NCT03541902.
References to ongoing studies
EUCTR2008‐000928‐71‐IT {published data only}
-
- EUCTR2007-002556-41-AT. A randomized, open-label, 2-arm, multicentre, phase II study to evaluate the safety and efficacy of trivax, a dendritic cell-based interleukin-12 secreting autologous cancer vaccine, in combination with sunitinib compared with sunitinib alone as first line treatment for patients with metastatic renal-cell carcinoma (mRCC). - Decavarec study TRX 1.0. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-002556-41-AT 2008.
-
- EUCTR2008-000928-71-IT. TWIST. Randomized prospective phase II study of temsirolimus with or without low-dose interferon alpha in metastatic non-clear renal cell carcinoma: GOIRC study 02/2008 - TWIST. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2008-000928-71-IT 2008.
-
- EUCTR2008-000928-71-IT. TWIST. Randomized prospective phase II study of temsirolimus with or without low-dose interferon alpha in metastatic non-clear renal cell carcinoma: GOIRC study 02/2008 - TWIST. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-000928-71-IT 2008.
NCT02210117 {published data only}
-
- Nivolumab with or without bevacizumab or ipilimumab before surgery in treating patients with metastatic kidney cancer that can be removed by surgery. clinicaltrials.gov/ct2/show/NCT02210117 2014. [ClinicalTrials.gov: NCT02210117]
NCT02996110 {published data only}
-
- A study to test combination treatments in people with advanced renal cell carcinoma (FRACTION-RCC). clinicaltrials.gov/ct2/show/NCT02996110 2016. [ClinicalTrials.gov: NCT02996110]
NCT03075423 {published data only}
-
- Ahrens M, Escudier B, Boleti E, Grimm MO, Goupil MG, Barthelemy P, et al. A randomized phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (suniforecast). Oncology Research and Treatment 2020;43 Suppl 1:226. [ClinicalTrials.gov: NCT03075423]
-
- Ahrens M, Escudier B, Haanen JB, Boleti E, Goupil MG, Grimm MO, et al. A randomized phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). In: Journal of Clinical Oncology. Meeting Abstract | 2021 ASCO Annual Meeting I. Vol. 39. 2021. [ClinicalTrials.gov: NCT03075423]
-
- Ahrens M, Escudier B, Haanen JB, Boleti E, Gross Goupil M, Grimm MO, et al. An ongoing, randomized phase II study of Nivolumab plus Ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). Oncology Research and Treatment 2021;44 Suppl 2:74-5. [ClinicalTrials.gov: NCT03075423]
-
- Ahrens M, Scheich S, Gokbuget N, Boleti E, Grunwald V, Escudier B, et al. A randomised phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). Oncology Research and Treatment 2018;41:73. [ClinicalTrials.gov: NCT03075423]
-
- Rexer H, Bergmann L, Steiner T. A phase 2, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in subjects with previously untreated and advanced (unresectable or metastatic) non-clear cell renal cell carcinoma - SUNNIFORECAST. Aktuelle Urologie 2020;51(3):236-8. [ClinicalTrials.gov: NCT03075423] - PubMed
NCT03260894 {published data only}
-
- NCT03260894. Pembrolizumab (MK-3475) plus epacadostat vs standard of care in mRCC (KEYNOTE-679/ECHO-302). clinicaltrials.gov/ct2/show/NCT03260894 2017. [ClinicalTrials.gov: NCT03260894]
NCT03592472 {published data only}
-
- Aggarwal RR, Thomas S, Hauke RJ, Nordquist LT, Munster PN. RENAVIV: a randomized phase III, double-blind, placebo-controlled study of pazopanib with or without abexinostat in patients with locally advanced or metastatic renal cell carcinoma. In: Journal of Clinical Oncology (Conference). Vol. 37. 2019. [ClinicalTrials.gov: NCT03592472]
NCT03729245 {published data only}
-
- NCT03729245. A study of bempegaldesleukin (NKTR-214: BEMPEG) in combination with nivolumab compared with the investigator's choice of a tyrosine kinase inhibitor (TKI) therapy (either sunitinib or cabozantinib monotherapy) for advanced metastatic renal cell carcinoma (RCC). clinicaltrials.gov/ct2/show/NCT03729245 2018. [ClinicalTrials.gov: NCT03729245]
-
- Tannir NM, Agarwal N, Pal SK, Cho DC, Formiga M, Guo J, et al. PIVOT-09: a phase III randomized open-label study of bempegaldesleukin (NKTR-214) plus nivolumab versus sunitinib or cabozantinib (investigator's choice) in patients with previously untreated advanced renal cell carcinoma (RCC). In: Journal of Clinical Oncology. Meeting Abstract | 2020 Genitourinary Cancers Symposium. Vol. 38. 2020. [ClinicalTrials.gov: NCT03729245]
NCT03793166 {published data only}
-
- Zhang T, Ballman K, Choudhury A, Chen R, Watt C, Wen Y, et al. PDIGREE: an adaptive phase 3 trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). Kidney Cancer 2020;4:S39. [ClinicalTrials.gov: NCT03793166]
-
- Zhang T, Ballman KV, Choudhury AD, Chen RC, Watt C, Wen Y, et al. PDIGREE: an adaptive phase 3 trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). In: Journal of Clinical Oncology. Meeting Abstract | 2021 Genitourinary Cancers Symposium. Vol. 37. 2019. [ClinicalTrials.gov: NCT03793166]
-
- Zhang T, Ballman KV, Choudhury AD, Chen RC, Watt C, Wen Y, et al. PDIGREE: an adaptive phase III trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). In: Journal of Clinical Oncology (Conference). Vol. 39. 2021. [ClinicalTrials.gov: NCT03793166]
NCT03873402 {published data only}
-
- An immunotherapy study of nivolumab plus ipilimumab versus nivolumab alone in participants with advanced kidney cancer. clinicaltrials.gov/show/NCT03873402 2019. [ClinicalTrials.gov: NCT03873402]
-
- NCT03873402. A study of nivolumab combined with ipilimumab versus nivolumab alone in participants with advanced kidney cancer. clinicaltrials.gov/ct2/show/NCT03873402 2019. [ClinicalTrials.gov: NCT03873402]
NCT03937219 {published data only}
-
- Choueiri T, Albiges L, Powles T, Geng A, Mohamed N, Wang F, et al. A phase 3 study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk. European Urology Open Science 2020;21Suppl 3:S188. [ClinicalTrials.gov: NCT03937219]
-
- Choueiri T, Albiges L, Powles T, Mohamed N, Wang F, Motzer R. A phase 3 study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk. Journal for Immunotherapy of Cancer 2020;8(3 Suppl):A209. [ClinicalTrials.gov: NCT03937219]
-
- Choueiri TK, Scheffold C, Wang F, Powles T, Motzer RJ. A phase 3 study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC) of intermediate or poor risk. Kidney Cancer 2020;4 Suppl 1:S2-3. [ClinicalTrials.gov: NCT03937219]
-
- De Giorgi U, Choueiri TK, Albiges L, Powles T, Scheffold C, Wang F, et al. A phase 3 study (Cosmic-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk. Tumori 2020;106(2 Suppl):152. [ClinicalTrials.gov: NCT03937219]
NCT04090710 {published data only}
-
- SBRT with combination ipilimumab/nivolumab for metastatic kidney cancer (CYTOSHRINK). clinicaltrials.gov/ct2/show/NCT04090710 2019. [ClinicalTrials.gov: NCT04090710]
NCT04203901 {published data only}
-
- Dendritic cell immunotherapy plus standard treatment of advanced renal cell carcinoma. clinicaltrials.gov/ct2/show/NCT04203901 2019. [ClinicalTrials.gov: NCT04203901]
NCT04394975 {published data only}
-
- Study to evaluate the efficacy and safety of toripalimab in combination with axitinib versus sunitinib monotherapy in advanced renal cell cancer. clinicaltrials.gov/ct2/show/NCT04394975 2020. [ClinicalTrials.gov: NCT04394975]
NCT04523272 {published data only}
-
- A study of TQB2450 injection combined with anlotinib hydrochloride capsule versus sunitinib in subjects with advanced renal cancer. clinicaltrials.gov/ct2/show/NCT04523272 2020. [ClinicalTrials.gov: NCT04523272]
NCT04540705 {published data only}
-
- A study to compare bempegaldesleukin (BEMPEG: NKTR-214) combined with nivolumab and tyrosine kinase inhibitor (TKI) to nivolumab and TKI alone in participants with previously untreated kidney cancer that is advanced or has spread (PIVOT IO 011). clinicaltrials.gov/ct2/show/NCT04540705 2020. [ClinicalTrials.gov: NCT04540705]
NCT04736706 {published data only}
-
- Choueiri T, Plimack E, Powles T, Voss M, Gurney H, Silverman R, et al. Phase 3 study of pembrolizumab + belzutifan + lenvatinib or pembrolizumab/quavonlimab + lenvatinib versus pembrolizumab + lenvatinib as first-line treatment for advanced renal cell carcinoma. Journal for ImmunoTherapy of Cancer 2021;9(2 Suppl):A447. [ClinicalTrials.gov: NCT04736706]
-
- EUCTR2020-002216-52-CZ. An open-label, randomized phase 3 study of immune and targeted combination therapies, as first-line treatment in participants with advanced clear cell renal cell carcinoma (ccRCC). trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-002216-52-CZ 2021. [ClinicalTrials.gov: NCT04736706]
-
- NCT04736706. A study of pembrolizumab (MK-3475) in combination with belzutifan (MK-6482) and lenvatinib (MK-7902), or pembrolizumab/quavonlimab (MK-1308A) in combination with lenvatinib, versus pembrolizumab and lenvatinib, for treatment of advanced clear cell renal cell carcinoma (MK-6482-012). clinicaltrials.gov/ct2/show/NCT04736706 2021. [ClinicalTrials.gov: NCT04736706]
-
- Rini BI, Plimack ER, Powles TB, Voss MH, Gurney HP, Silverman R, et al. 717TiP randomized, open-label, 3-arm phase III study comparing MK-1308A + lenvatinib and pembrolizumab (pembro) + belzutifan + lenvatinib versus pembro + lenvatinib as first-line (1L) treatment for advanced clear cell renal cell carcinoma (ccRCC). Annals of Oncology 2021;32 Suppl 5:S722-3. [ClinicalTrials.gov: NCT04736706]
NCT05043090 {published data only}
-
- EUCTR2021-000336-55-ES. A clinical trial to compare the effectiveness of savolitinib plus durvalumab relative to sunitinib for the treatment of renal cell cancer. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-000336-55-ES 2021.
-
- NCT05043090. Savolitinib plus Durvalumab versus sunitinib and Durvalumab monotherapy in MET-Driven, unresectable and locally advanced or metastatic PRCC. clinicaltrials.gov/ct2/show/NCT05043090 2021.
NCT05096390 {published data only}
-
- Axitinib +/- Pembrolizumab in first line treatment of mPRCC. clinicaltrials.gov/ct2/show/NCT05096390 2021. [ClinicalTrials.gov: NCT05096390]
UMIN 000012522 {published data only}
-
- Kadono Y, Konaka H, Izumi K, Anai S, Fujimoto K, Ishibashi K, et al. Efficacy and safety of cytokines versus first-line sunitinib and second-line axitinib for patients with metastatic renal cell carcinoma (ESCAPE study): a study protocol for phase III randomized sequential open-label study. Contemporary Clinical Trials Communications 2019;15:1-6. - PMC - PubMed
-
- UMIN000012522. Efficacy and safety of cytokines versus sunitinib as first-line followed by second-line axitinib in the treatment of patients with metastatic renal cell carcinoma: a phase III randomized sequential open-label study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014557 2013.
Additional references
Aguiar 2018
-
- Aguiar P, Costa de Padua T, Noia Barreto CM, Del Giglio A. Treatment of metastatic renal cell carcinoma: latest evidence and ongoing challenges. Clinical Medicine Insights: Urology 2018;11:1-7.
Aldin 2023
-
- Aldin A. First-line therapy for adults with advanced renal cell carcinoma - supplementary file. Open Science Framework 2023. [DOI: 10.17605/OSF.IO/HXBG5] - DOI - PMC - PubMed
American Cancer Society 2022
-
- Cancer Facts & Figures 2022. Atlanta: American Cancer Society 2022.
ASCO 2021
-
- American Society of Clinical Oncology. Kidney cancer: introduction. https://www.cancer.net/cancer-types/kidney-cancer/introduction#:~:text=R.... (accessed 21 December 2022).
ASCO 2022
-
- American Society of Clinical Oncology. Kidney Cancer: Statistics. https://www.cancer.net/cancer-types/kidney-cancer/statistics (accessed 21 December 2022).
Bosma 2022
Brierley 2016
-
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th edition. Oxford: John Wiley & Sons, 2016.
Capitanio 2019
Cattrini 2021
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. - PubMed
Chaimani 2013
Chaimani 2019
-
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Choueiri 2017b
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. New England Journal of Medicine 2017;376(4):354-66. - PubMed
CINeMA [Computer program]
-
- CINeMA [Confidence in Network Meta-Analysis]. Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al, Version (accessed prior to 22 October 2020). The University of Bern, The Campbell Collaboration, The Cochrane Collaboration, 2020. cinema.ispm.unibe.ch/.
Coppin 2004
Dabestani 2016
-
- Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World Journal of Urology 2016;34(8):1081-6. - PubMed
de Groot 2018
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane 2022. Available from www.training.cochrane.org/handbook 2022.
Delahunt 2019
-
- Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology 2019;74(1):4-17. - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. - PubMed
Edwards 2018
-
- Edwards SJ, Wakefield V, Cain P, Karner C, Kew K, Bacelar M, et al. Axitinib, cabozantinib, everolimus, nivolumab, sunitinib and best supportive care in previously treated renal cell carcinoma: a systematic review and economic evaluation. Health Technology Assessment 2018;22(6):1-278. - PMC - PubMed
Egger 1997
Elliott 2014
Escudier 2019
-
- Escudier B, Porta C, Schmidinger M, Rioux-Leclerq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019;30(5):706-20. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. - PubMed
Garner 2016
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 9 22 October 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). gradepro.org.
Guyatt 2017
-
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: Assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. - PubMed
Harshman 2014
Higgins 2019
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Higgins 2019a
-
- Higgins JP, Eldridge S, Li T. Chapter 23: including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Higgins 2019b
-
- Higgins JP, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Higgins 2019c
-
- Higgins JP, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16: special topics in statistics. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Keir 2007
-
- Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Annual Review of Immunology 2007;19(3):309-14. - PubMed
Krahn 2013
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Leitlinienprogramm Onkologie
-
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). Diagnostics, therapy and aftercare of renal cell carcinoma - Longversion 3.0 [Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms - Langversion 3.0]. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downlo....
Li 2019
-
- Li T, Higgins JP, Deeks JJ. Chapter 5: collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Liu 2021
-
- Liu Z, Chen Y, Wei Z, He Y, Wang J, Mu X, et al. Comparative efficacy and safety of immunotherapy in the first-line treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Annals of Palliative Medicine 2021;10(3):2805-14. - PubMed
Ljungberg 2022
-
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. European Urology 2022;82(4):399–410. - PubMed
Lopez‐Beltran 2009
-
- Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. International Journal of Urology 2009;16(5):432-43. - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. - PubMed
Mori 2021
Motzer 2022
Nassar 2022
Nocera 2022
-
- Nocera L, Karakiewicz P I, Wenzel M, Tian Z, Shariat S F, Saad F, et al. Clinical outcomes and adverse events after first-line.treatment in metastatic renal cell carcinoma: a systematic review and network meta-analysis. Journal of Urology 2022;207(1):16-24. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. - PubMed
Powles 2021
-
- Powles T, Albiges L, Bex A, Grünwald V, Porta C, Procopio G, et al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Annals of Oncology 2021;32(12):1511-9. - PubMed
Puhan 2014
Qin 2018
Quhal 2021
-
- Quhal F, Mori K, Bruchbacher A, Resch I, Mostafaei H, Pradere B, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. European Urology Oncology 2021;4(5):755-65. - PubMed
R Core Team 2019 [Computer program]
-
- R: A language and environment for statistical computing. R Core Team 2019. Vienna, Austria: R Foundation for Statistical Computing, 2019. Available at www.R-project.org.
Riaz 2021
-
- Riaz IB, He H, Ryu AJ, Siddiqi R, Naqvi SA, Yao Y, et al. A living, interactive systematic review and network meta-analysis of first-line treatment of metastatic renal cell carcinoma. European Urology 2021;80(6):712-23. - PubMed
Rini 2009
-
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373(9669):1119-32. - PubMed
Robert Koch Institute 2021
-
- Robert Koch Institute. Cancer in Germany in 2017/2018 [Krebs in Deutschland für 2017/2018]. https://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_German... 2021.
Rücker 2012
-
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. - PubMed
Rücker 2014
-
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. - PubMed
Rücker 2015
Rücker 2019
-
- Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G. netmeta: Network meta-analysis using frequentist methods. R package version 1.10. CRAN.R-project.org/package=netmeta 2019.
Sabatini 2006
-
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature Reviews Cancer 2006;6(9):729-34. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. - PubMed
Salanti 2014
Sanchez‐Gastaldo 2017
-
- Sanchez-Gastaldo A, Kempf E, Gonzalez del Alba A, Duran I. Systemic treatment of renal cell cancer: a comprehensive review. Cancer Treatment Reviews 2017;60:77-89. - PubMed
Scelo 2018
Schwarzer 2007
-
- Schwarzer G. meta: an R package for meta-analysis. R News 2007;7(3):40-5.
Schwarzer 2015
-
- Schwarzer G, Carpenter JR, Rücker G. Chapter 8: network meta-analysis. In: Meta-Analysis With R. Springer International Publishing Switzerland, 2015.
Schünemann 2019
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Siegel 2022
-
- Siegel RL, Miller KD, Fuchs H, Jemal A. Cancer statistics 2022. CA: A Cancer Journal for Clinicians 2022;72(1):7-33. - PubMed
Skoetz 2019
-
- Skoetz N, Goldkuhle M, Weigl A, Dwan K, Labonte V, Dahm P, et al. Methodological review showed correct absolute effect size estimates for time-to-event outcomes in less than one-third of cancer-related systematic reviews. Journal of Clinical Epidemiology 2019;108:1-9. - PubMed
Skoetz 2020
-
- Skoetz N, Goldkuhle M, Dalen EC, Akl EA, Trivella M, Mustafa R, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. - PubMed
Sterne 2019
-
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. - PubMed
Sun 2011
-
- Sun M, Thuret R, Abdollah F, Lughezzani G, Schmitges J, Tian Z, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. European Urology 2011;59(1):135-41. - PubMed
Swallow 2018
-
- Swallow E, Messali A, Ghate S, McDonald E, Duchesneau E, Perez JR. The additional costs per month of progression-free survival and overall survival: an economic model comparing everolimus with cabozantinib, nivolumab, and axitinib for second-line treatment of metastatic renal cell carcinoma. Journal of Managed Care & Specialty Pharmacy 2018;24(4):335-43. - PMC - PubMed
Thekdi 2015
Tierney 2007
Tudur 2001
-
- Tudur C, Williamson PR, Khan S, Best LY. The value of the aggregate data approach in meta-analysis with time-to-event outcomes. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2001;164(2):357-70.
UICC 2017
-
- Union for International Cancer Control (UICC). TNM Classification of Malignant Tumours. 8th edition. Wiley-Blackwell, 2017.
United States Congress 2007
-
- United States Congress 2007. Food and Drug Administration Amendments Act (FDAAA) of 2007: public law no 110-85. govinfo.gov/content/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf.
Unverzagt 2017
Wang 2019
Warren 2018
White 2012
World Medical Association
-
- World Medical Association. World Medical Association Declaration of Helsinki - ethical principles for medical research involving human subjects. Journal of the American Medical Association 2013;310(20):2191-4. - PubMed
References to other published versions of this review
Goldkuhle 2020
-
- Goldkuhle M, Aldin A, Jakob T, Adams A, Monsef I, Heidenreich A, et al. First‐line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD013798. [DOI: 10.1002/14651858.CD013798] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

