Prebiotic Synthesis of N- Formylaminonitriles and Derivatives in Formamide
- PMID: 37146260
- PMCID: PMC10197134
- DOI: 10.1021/jacs.2c13306
Prebiotic Synthesis of N- Formylaminonitriles and Derivatives in Formamide
Abstract
Amino acids and their derivatives were probably instrumental in the transition of prebiotic chemistry to early biology. Accordingly, amino acid formation under prebiotic conditions has been intensively investigated. Unsurprisingly, most of these studies have taken place with water as the solvent. Herein, we describe an investigation into the formation and subsequent reactions of aminonitriles and their formylated derivatives in formamide. We find that N-formylaminonitriles form readily from aldehydes and cyanide in formamide, even in the absence of added ammonia, suggesting a potentially prebiotic source of amino acid derivatives. Alkaline processing of N-formylaminonitriles proceeds with hydration at the nitrile group faster than deformylation, protecting aminonitrile derivatives from reversion of the Strecker condensation equilibrium during hydration/hydrolysis and furnishing mixtures of N-formylated and unformylated amino acid derivatives. Furthermore, the facile synthesis of N-formyldehydroalanine nitrile is observed in formamide from glycolaldehyde and cyanide without intervention. Dehydroalanine derivatives have been proposed as important compounds for prebiotic peptide synthesis, and we demonstrate both a synthesis suggesting that they are potentially plausible components of a prebiotic inventory, and reactions showing their utility as abiotic precursors to a range of compounds of prebiological interest.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

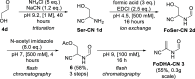
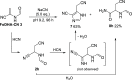

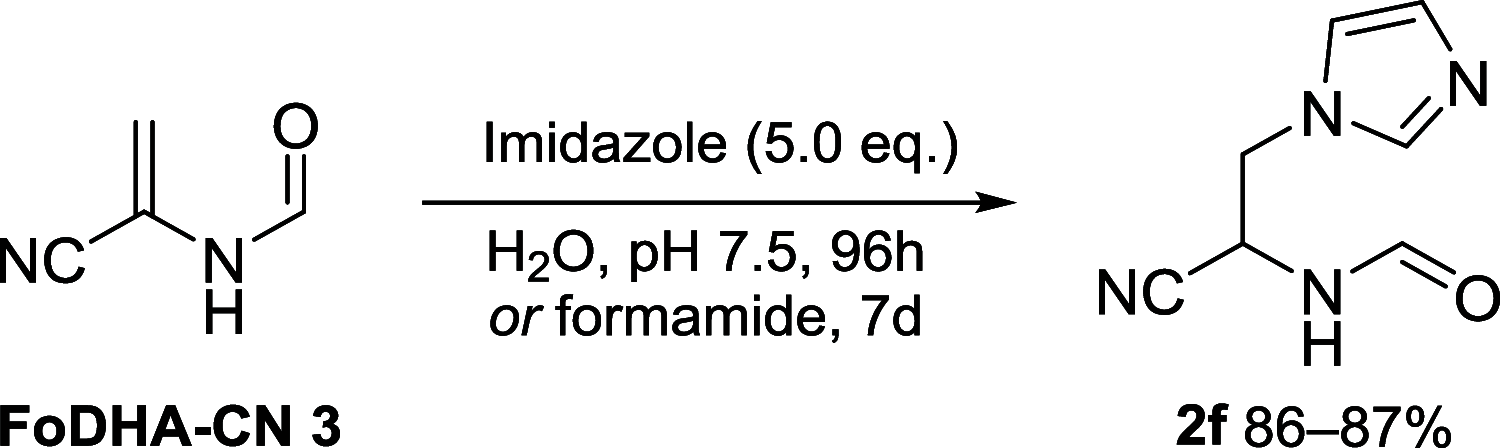
References
-
- Pascal R.; Boiteau L.; Commeyras A.. From the Prebiotic Synthesis of α-Amino Acids Towards a Primitive Translation Apparatus for the Synthesis of Peptides. Prebiotic Chemistry; Springer-Verlag: Berlin: pp 69–122.
-
- Strecker A. Ueber Die Künstliche Bildung Der Milchsäure Und Einen Neuen, Dem Glycocoll Homologen Körper. Ann. Chem. Pharm. 1850, 75, 27–45. 10.1002/jlac.18500750103. - DOI
-
- Bergs H.Hydantoin Derivatives. Patent DE566094, 1932.
-
- Hafenbradl D.; Keller M.; Wächtershäuser G.; Stetter K. O. Primordial Amino Acids by Reductive Amination of α-Oxo Acids in Conjunction with the Oxidative Formation of Pyrite. Tetrahedron Lett. 1995, 36, 5179–5182. 10.1016/0040-4039(95)01008-6. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

