Deriving Schwann cells from hPSCs enables disease modeling and drug discovery for diabetic peripheral neuropathy
- PMID: 37146583
- PMCID: PMC10249419
- DOI: 10.1016/j.stem.2023.04.006
Deriving Schwann cells from hPSCs enables disease modeling and drug discovery for diabetic peripheral neuropathy
Abstract
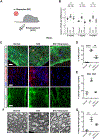
Schwann cells (SCs) are the primary glia of the peripheral nervous system. SCs are involved in many debilitating disorders, including diabetic peripheral neuropathy (DPN). Here, we present a strategy for deriving SCs from human pluripotent stem cells (hPSCs) that enables comprehensive studies of SC development, physiology, and disease. hPSC-derived SCs recapitulate the molecular features of primary SCs and are capable of in vitro and in vivo myelination. We established a model of DPN that revealed the selective vulnerability of SCs to high glucose. We performed a high-throughput screen and found that an antidepressant drug, bupropion, counteracts glucotoxicity in SCs. Treatment of hyperglycemic mice with bupropion prevents their sensory dysfunction, SC death, and myelin damage. Further, our retrospective analysis of health records revealed that bupropion treatment is associated with a lower incidence of neuropathy among diabetic patients. These results highlight the power of this approach for identifying therapeutic candidates for DPN.
Keywords: demyelination; drug repurposing; metabolic stress; myelination disorders; nerve repair.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests F.F., L.S., S.C., and Z.G. are inventors on several patent applications owned by UCSF, MSKCC, and Weill Cornell Medicine related to hPSC differentiation technologies including the technologies reported in this manuscript. L.S. is a scientific co-founder and paid consultant of BlueRock Therapeutics and DaCapo BrainScience.
Figures





Comment in
-
hPSCs-derived Schwann cells serve for disease modeling and drug discovery.Cell Stem Cell. 2023 May 4;30(5):501-502. doi: 10.1016/j.stem.2023.04.013. Cell Stem Cell. 2023. PMID: 37146574
References
-
- Lavdas AA, Papastefanaki F, Thomaidou D, and Matsas R (2008). Schwann cell transplantation for CNS repair. Curr. Med. Chem. 15, 151–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

