The mitochondrial unfolded protein response regulates hippocampal neural stem cell aging
- PMID: 37146607
- PMCID: PMC10330239
- DOI: 10.1016/j.cmet.2023.04.012
The mitochondrial unfolded protein response regulates hippocampal neural stem cell aging
Abstract
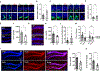
Aging results in a decline in neural stem cells (NSCs), neurogenesis, and cognitive function, and evidence is emerging to demonstrate disrupted adult neurogenesis in the hippocampus of patients with several neurodegenerative disorders. Here, single-cell RNA sequencing of the dentate gyrus of young and old mice shows that the mitochondrial protein folding stress is prominent in activated NSCs/neural progenitors (NPCs) among the neurogenic niche, and it increases with aging accompanying dysregulated cell cycle and mitochondrial activity in activated NSCs/NPCs in the dentate gyrus. Increasing mitochondrial protein folding stress results in compromised NSC maintenance and reduced neurogenesis in the dentate gyrus, neural hyperactivity, and impaired cognitive function. Reducing mitochondrial protein folding stress in the dentate gyrus of old mice improves neurogenesis and cognitive function. These results establish the mitochondrial protein folding stress as a driver of NSC aging and suggest approaches to improve aging-associated cognitive decline.
Keywords: SIRT1; SIRT2; SIRT3; SIRT6; SIRT7; cognitive aging; mitochondrial unfolded protein response; neural stem cell aging; sirtuin; stem cell aging.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






Comment in
-
Unfolding cognitive decline mechanisms.Nat Rev Neurosci. 2023 Jul;24(7):394. doi: 10.1038/s41583-023-00715-8. Nat Rev Neurosci. 2023. PMID: 37268821 No abstract available.

