ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines
- PMID: 37146611
- PMCID: PMC10121106
- DOI: 10.1016/j.cell.2023.04.024
ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines
Abstract
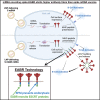
Prime-boost regimens for COVID-19 vaccines elicit poor antibody responses against Omicron-based variants and employ frequent boosters to maintain antibody levels. We present a natural infection-mimicking technology that combines features of mRNA- and protein nanoparticle-based vaccines through encoding self-assembling enveloped virus-like particles (eVLPs). eVLP assembly is achieved by inserting an ESCRT- and ALIX-binding region (EABR) into the SARS-CoV-2 spike cytoplasmic tail, which recruits ESCRT proteins to induce eVLP budding from cells. Purified spike-EABR eVLPs presented densely arrayed spikes and elicited potent antibody responses in mice. Two immunizations with mRNA-LNP encoding spike-EABR elicited potent CD8+ T cell responses and superior neutralizing antibody responses against original and variant SARS-CoV-2 compared with conventional spike-encoding mRNA-LNP and purified spike-EABR eVLPs, improving neutralizing titers >10-fold against Omicron-based variants for 3 months post-boost. Thus, EABR technology enhances potency and breadth of vaccine-induced responses through antigen presentation on cell surfaces and eVLPs, enabling longer-lasting protection against SARS-CoV-2 and other viruses.
Keywords: ESCRT; SARS-CoV-2; mRNA vaccines; nanoparticles.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.A.G.H. and P.J.B. are inventors on a US patent application filed by the California Institute of Technology that covers the EABR technology described in this work. W.J.M. and P.J.C.L. are employees of Acuitas Therapeutics, a company developing LNP delivery technology; P.J.C.L. holds equity in Acuitas Therapeutics.
Figures





Update of
-
ESCRT recruitment to mRNA-encoded SARS-CoV-2 spike induces virus-like particles and enhanced antibody responses.bioRxiv [Preprint]. 2022 Dec 27:2022.12.26.521940. doi: 10.1101/2022.12.26.521940. bioRxiv. 2022. Update in: Cell. 2023 May 25;186(11):2380-2391.e9. doi: 10.1016/j.cell.2023.04.024. PMID: 36597535 Free PMC article. Updated. Preprint.
Comment in
-
A budding new approach strengthens an important message.Cell. 2023 May 25;186(11):2283-2285. doi: 10.1016/j.cell.2023.04.034. Cell. 2023. PMID: 37236153 Free PMC article.
-
Two-birds-one-stone approach to combine protein and mRNA vaccines for COVID-19.Nat Immunol. 2023 Jul;24(7):1056-1057. doi: 10.1038/s41590-023-01539-5. Nat Immunol. 2023. PMID: 37337102 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

