Plasma membrane preassociation drives β-arrestin coupling to receptors and activation
- PMID: 37146613
- PMCID: PMC7614532
- DOI: 10.1016/j.cell.2023.04.018
Plasma membrane preassociation drives β-arrestin coupling to receptors and activation
Abstract
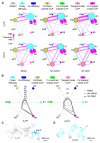
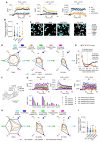
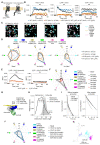
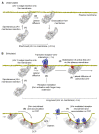
β-arrestin plays a key role in G protein-coupled receptor (GPCR) signaling and desensitization. Despite recent structural advances, the mechanisms that govern receptor-β-arrestin interactions at the plasma membrane of living cells remain elusive. Here, we combine single-molecule microscopy with molecular dynamics simulations to dissect the complex sequence of events involved in β-arrestin interactions with both receptors and the lipid bilayer. Unexpectedly, our results reveal that β-arrestin spontaneously inserts into the lipid bilayer and transiently interacts with receptors via lateral diffusion on the plasma membrane. Moreover, they indicate that, following receptor interaction, the plasma membrane stabilizes β-arrestin in a longer-lived, membrane-bound state, allowing it to diffuse to clathrin-coated pits separately from the activating receptor. These results expand our current understanding of β-arrestin function at the plasma membrane, revealing a critical role for β-arrestin preassociation with the lipid bilayer in facilitating its interactions with receptors and subsequent activation.
Keywords: G protein-coupled receptors; GPCR; TIRF; arrestin; plasma membrane; protein-protein interactions; single-molecule microscopy.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
GPCRs and β-arrestins - an on-off relationship.Cell Res. 2023 Nov;33(11):819-820. doi: 10.1038/s41422-023-00838-8. Cell Res. 2023. PMID: 37337029 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

