Belimumab versus anifrolumab in adults with systemic lupus erythematosus: an indirect comparison of clinical response at 52 weeks
- PMID: 37147022
- PMCID: PMC10186457
- DOI: 10.1136/lupus-2023-000907
Belimumab versus anifrolumab in adults with systemic lupus erythematosus: an indirect comparison of clinical response at 52 weeks
Abstract
Objective: To generate comparative efficacy evidence of belimumab versus anifrolumab in SLE that can inform treatment practices.
Methods: The SLE Responder Index (SRI)-4 response at 52 weeks of belimumab versus anifrolumab was evaluated with an indirect treatment comparison. The evidence base consisted of randomised trials that were compiled through a systemic literature review.A feasibility assessment was performed to comprehensively compare the eligible trials and to determine the most appropriate indirect treatment comparison analysis method. A multilevel network meta-regression (ML-NMR) was implemented that adjusted for differences across trials in four baseline characteristics: SLE Disease Activity Index-2K, anti-double-stranded DNA antibody positive, low complement (C)3 and low C4. Additional analyses were conducted to explore if the results were robust to different sets of baseline characteristics included for adjustment, alternative adjustment methods and changes to the trials included in the evidence base.
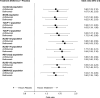
Results: The ML-NMR included eight trials: five belimumab trials (BLISS-52, BLISS-76, NEA, BLISS-SC, EMBRACE) and three anifrolumab trials (MUSE, TULIP-1, TULIP-2). Belimumab and anifrolumab were comparable in terms of SRI-4 response (OR (95% credible interval), 1.04 (0.74-1.45)), with the direction of the point estimate slightly favouring belimumab. Belimumab had a 0.58 probability of being the more effective treatment. The results were highly consistent across all analysis scenarios.
Conclusions: Our results suggest that the SRI-4 response of belimumab and anifrolumab are similar at 52 weeks in the general SLE population, but the level of uncertainty around the point estimate means we cannot rule out the possibility of a clinically meaningful benefit for either treatment. It remains to be seen if specific groups of patients could derive a greater benefit from anifrolumab or from belimumab, and there is certainly an unmet need to identify robust predictors towards more personalised selection of available biological agents in SLE.
Keywords: Antirheumatic Agents; Biological Products; Outcome Assessment, Health Care; Therapeutics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BN, PS, MS and AM are employees of Evidera, a part of Thermo Fisher Scientific. MP has received grant/research support from AstraZeneca, Aurinia, Eli Lilly, Exagen, GSK, Janssen and Thermo Fisher. MP has also received consulting fees from the BPR Scientific Advisory Committee, Alexion, Amgen, AnaptysBio, Argenx, AstraZeneca, Aurinia, AxDev, Biogen, Boston Pharmaceuticals, Caribou Biosciences, CVS Health, Eli Lilly, Gilead Biosciences, GSK, Idorsia Pharmaceuticals, Janssen, Kezar Life Sciences, Kira Pharmaceuticals, Momenta Pharmaceuticals, Nimbus Lakshmi, Proviant, Sanofi, SinoMab and UCB. MP has received speakers’ fees from Aurinia, MedShr and Arthros-FocusMedEd, and has received consulting fees for participation in a data safety monitoring board or advisory board for EMD Serono, Emergent Biosolutions, IQVIA and PPD Development. GKB has received consulting fees from Pfizer, Lilly and Novartis, and has received honorary fees from GSK, AstraZeneca, Pfizer, Novartis, Aenorasis, AbbVie and Lilly. GKB has also received a research grant from Pfizer. AHJK has received research support to Washington University from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number P30 AR073752), National Center for Advancing Translational Sciences (grant number UL1 TR002345), Leona M. and Harry B. Helmsley Charitable Trust, Rheumatology Research Foundation, and National Multiple Sclerosis Society, GSK, and Foghorn Therapeutics. AHJK has performed consultancy for Alexion Pharmaceuticals, ANI Pharmaceuticals, AstraZeneca, Aurinia Pharmaceuticals, Exagen Diagnostics, GSK, Kypha and Pfizer unrelated to this work. AHJK has received payment or honoraria (for lectures, presentations, speakers bureaus, manuscript writing or educational events) from AstraZeneca, Aurinia Pharmaceuticals, Exagen Diagnostics and GSK. AHJK has participated on a data safety monitoring board or advisory board for National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. AHJK has been a board member for the Rheumatology Research Foundation Scientific Advisory Board and the Lupus Foundation of America-Heartland Chapter, and president of the St Louis Rheumatology Association. AHJK is the inventor of patent number 11029318 with Kypha unrelated to this work. The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Washington University, its affiliated academic health care centers, or the National Institutes of Health. AF has received honoraria and consulting fees from GSK, Aenorasis and AstraZeneca. AF has been a paid speaker for AbbVie, Amgen, Pfizer, Lilly, Genesis-Pharma, Novartis, UCB and Boehringer-Ingelheim. RAL, DC and NB are employees of GSK and hold stocks and shares in GSK.
Figures


References
-
- GSK . Benlysta us prescribing information. Available: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Inform... [Accessed Jul 2022].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
