Parapneumonic effusions related to Streptococcus pneumoniae: serotype and disease severity trends from 2006 to 2018 in Bristol, UK
- PMID: 37147024
- PMCID: PMC10163460
- DOI: 10.1136/bmjresp-2022-001440
Parapneumonic effusions related to Streptococcus pneumoniae: serotype and disease severity trends from 2006 to 2018 in Bristol, UK
Abstract
Rationale: Streptococcus pneumoniae epidemiology is changing in response to vaccination and some data suggest that empyema incidence is increasing. However, differences exist between the UK and US studies. We describe trends in the clinical phenotype of adult pneumococcal pleural infection, including simple parapneumonic effusions (SPE) in the pneumococcal conjugate vaccination (PCV) era.
Objectives: To determine whether there were differences in pneumococcal disease presentation and severity associated with pleural infection.
Methods: A retrospective cohort study, all adults ≥16 years admitted to three large UK hospitals, 2006-2018 with pneumococcal disease. 2477 invasive pneumococcal cases were identified: 459 SPE and 100 pleural infection cases. Medical records were reviewed for each clinical episode. Serotype data were obtained from the UK Health Security Agency national reference laboratory.
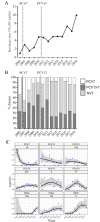
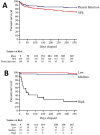
Results: Incidence increased over time, including non-PCV-serotype disease. PCV7-serotype disease declined following paediatric PCV7 introduction, but the effect of PCV13 was less apparent as disease caused by the additional six serotypes plateaued with serotypes 1 and 3 causing such parapneumonic effusions from 2011 onwards.Patients with pleural infection had a median survival 468 days (95% CI 340 to 590) vs 286 days (95% CI 274 to 335) in those with SPE. Pleural infection associated with frank pus had lower 90-day mortality than pleural infection without pus (0% vs 29%, p<0.0001). 90-day mortality could be predicted by baseline increased RAPID (Renal, Age, Purulence, Infection source, and Dietary factors) score (HR 15.01, 95% CI 1.24 to 40.06, p=0.049).
Conclusions: Pneumococcal infection continues to cause severe disease despite the introduction of PCVs. The predominance of serotype 1 and 3 in this adult UK cohort is in keeping with previous studies in paediatric and non-UK studies. Rising non-PCV serotype disease and limited impact of PCV13 on cases caused by serotypes 1 and 3 offset the reductions in adult pneumococcal parapneumonic effusion disease burden observed following the introduction of the childhood PCV7 programme.
Keywords: bacterial infection; empyema; pleural disease; respiratory infection.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CH is the principal investigator of the AvonCAP study which is an investigator-led University of Bristol study funded by Pfizer. AF is a member of the Joint Committee on Vaccination and Immunization (JCVI) and chair of the World Health Organization European Technical Advisory Group of Experts on Immunization (ETAGE) committee. In addition to receiving funding from Pfizer as chief investigator of this study, he leads another project investigating transmission of respiratory bacteria in families jointly funded by Pfizer and the Gates Foundation.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
