Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells
- PMID: 37147112
- PMCID: PMC10359520
- DOI: 10.1136/ard-2023-223952
Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells
Keywords: B-Lymphocytes; Systemic Sclerosis; Treatment.
Conflict of interest statement
Competing interests: None declared.
Figures
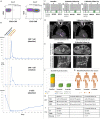
References
-
- Maher TM, Tudor VA, Saunders P, et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): A double-blind, double-dummy, randomised, controlled, phase 2B trial. Lancet (London, England) 2023;11:45–54. 10.1016/S2213-2600(22)00359-9 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

