Prognostic Value of Persistent CSF Antibodies at 12 Months in Anti-NMDAR Encephalitis
- PMID: 37147137
- PMCID: PMC10162705
- DOI: 10.1212/NXI.0000000000200108
Prognostic Value of Persistent CSF Antibodies at 12 Months in Anti-NMDAR Encephalitis
Abstract
Background and objectives: Anti-NMDA receptor (NMDAR) encephalitis is defined by the presence of antibodies (Abs) targeting the NMDAR in the CSF. This study aimed to determine the prognostic value of persistent CSF NMDAR-Abs during follow-up.
Methods: This retrospective observational study included patients diagnosed with anti-NMDAR encephalitis in the French Reference Center for Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis and for whom CSF samples were obtained at diagnosis and >4 months of follow-up to evaluate CSF NMDAR-Ab persistence. Because patients were tested for CSF NMDAR-Abs at different time points, samples were stratified into different periods of follow-up (i.e., 12 months was considered for the 9- to 16-month follow-up period).
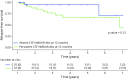
Results: Among the 501 patients diagnosed with anti-NMDAR encephalitis between January 2007 and June 2020, 89 (17%) were tested between 4 and 120 months for CSF NMDAR-Abs after clinical improvement and included in the study (75/89 women, 84%; median age 20 years, interquartile range [IQR] 16-26). During follow-up, 21 of 89 (23%) patients had a relapse after a median time of 29 months (IQR 18-47), and 20 of 89 (22%) had a poor outcome (mRS ≥3) after a median last follow-up of 36 months (IQR 19-64). Most patients (69/89, 77%) were tested at the 12-month follow-up period, and 42 of 69 (60%) of them had persistent CSF NMDAR-Abs. When comparing patients with persistent or absent CSF NMDAR-Abs at 12 months, poor outcome at the last follow-up was more frequent in the former (38% vs 8%, p = 0.01), who had relapses more often (23% vs 7%), which also appeared earlier in the course of the disease (90% during the following 4 years of follow-up vs 20%), although no significant difference was observed at long-term follow-up (p = 0.15). In addition, patients with persistent CSF NMDAR-Abs at 12 months had higher titers of CSF NMDAR-Abs at diagnosis.
Discussion: In this study, patients with persistent CSF NMDAR-Abs at 12 months were more likely to have subsequent relapses and a poor long-term outcome. However, these findings should be interpreted with caution because of the variability in the time of sampling of this study. Future prospective studies are required to validate these results in larger cohorts.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
The authors have no disclosures relevant to the manuscript. Go to
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
