An inducible model for genetic manipulation and fate-tracing of PDGFRβ-expressing fibrogenic cells in the liver
- PMID: 37147343
- PMCID: PMC10162963
- DOI: 10.1038/s41598-023-34353-y
An inducible model for genetic manipulation and fate-tracing of PDGFRβ-expressing fibrogenic cells in the liver
Abstract
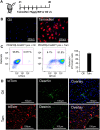
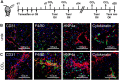
Myofibroblasts are the source of extracellular matrix protein during liver fibrogenesis. Fibroblasts, hepatic stellate cells (HSCs) and vascular smooth muscle cells are mesenchymal subpopulations in the liver that are characterized by the expression of PDGFRβ and contribute to the pool of these myofibroblasts. Conditional knockout models are important to better understand the function of specific liver cell populations including mesenchymal cells. While there is a limited number of constitutive mouse models for liver mesenchymal cell specific transgene expression, there is no established model for inducible gene targeting in HSCs or PDGFRβ-expressing mesenchymal cell populations in the liver. To address this, we investigated whether the tamoxifen inducible PDGFRβ-P2A-CreERT2 mouse can be used as a reliable tool to specifically express transgens in liver mesenchymal cells. Our data demonstrate, that PDGFRβ-P2A-CreERT2 specifically and efficiently marks over 90% of retinoid positive HSCs in healthy and fibrotic liver in mice upon tamoxifen injection, and that those cells give rise to Col1a1-expressing myofibroblasts in different models of liver fibrosis. Together with a negligible background recombination of only about 0.33%, this confirms that the PDGFRβ-P2A-CreERT2 mouse is nearly as efficient as established constitutive LratCre and PDGFRβ-Cre mouse models for recombination in HSCs, and that it is a powerful model for mesenchymal liver cell studies that require an inducible Cre approach.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Roth GA, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

