Initiating angiotensin II at lower vasopressor doses in vasodilatory shock: an exploratory post-hoc analysis of the ATHOS-3 clinical trial
- PMID: 37147690
- PMCID: PMC10163684
- DOI: 10.1186/s13054-023-04446-1
Initiating angiotensin II at lower vasopressor doses in vasodilatory shock: an exploratory post-hoc analysis of the ATHOS-3 clinical trial
Abstract
Background: High dose vasopressors portend poor outcome in vasodilatory shock. We aimed to evaluate the impact of baseline vasopressor dose on outcomes in patients treated with angiotensin II (AT II).
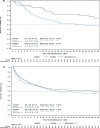
Methods: Exploratory post-hoc analysis of the Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) trial data. The ATHOS-3 trial randomized 321 patients with vasodilatory shock, who remained hypotensive (mean arterial pressure of 55-70 mmHg) despite receiving standard of care vasopressor support at a norepinephrine-equivalent dose (NED) > 0.2 µg/kg/min, to receive AT II or placebo, both in addition to standard of care vasopressors. Patients were grouped into low (≤ 0.25 µg/kg/min; n = 104) or high (> 0.25 µg/kg/min; n = 217) NED at the time of study drug initiation. The primary outcome was the difference in 28-day survival between the AT II and placebo subgroups in those with a baseline NED ≤ 0.25 µg/kg/min at the time of study drug initiation.
Results: Of 321 patients, the median baseline NED in the low-NED subgroup was similar in the AT II (n = 56) and placebo (n = 48) groups (median of each arm 0.21 µg/kg/min, p = 0.45). In the high-NED subgroup, the median baseline NEDs were also similar (0.47 µg/kg/min AT II group, n = 107 vs. 0.45 µg/kg/min placebo group, n = 110, p = 0.75). After adjusting for severity of illness, those randomized to AT II in the low-NED subgroup were half as likely to die at 28-days compared to placebo (HR 0.509; 95% CI 0.274-0.945, p = 0.03). No differences in 28-day survival between AT II and placebo groups were found in the high-NED subgroup (HR 0.933; 95% CI 0.644-1.350, p = 0.71). Serious adverse events were less frequent in the low-NED AT II subgroup compared to the placebo low-NED subgroup, though differences were not statistically significant, and were comparable in the high-NED subgroups.
Conclusions: This exploratory post-hoc analysis of phase 3 clinical trial data suggests a potential benefit of AT II introduction at lower doses of other vasopressor agents. These data may inform design of a prospective trial.
Trial registration: The ATHOS-3 trial was registered in the clinicaltrials.gov repository (no. NCT02338843). Registered 14 January 2015.
Keywords: Angiotensin; Catecholamine; Multimodal; Outcomes; Shock; Vasopressor.
© 2023. The Author(s).
Conflict of interest statement
RB, KRH, and AMD, report no conflicts of interest. JHC, PMW, AKK, RGW, and DWB have previously served on a scientific advisory board for La Jolla Pharmaceutical Company. JHC, LWB, AKK, and DWB have served on the Speaker’s Bureau for La Jolla Pharmaceutical Company. AZ and JHC have received consultant fees from La Jolla Pharmaceutical Company. AZ has received consultant fees from Paion. AKK received research grant funding from La Jolla Pharmaceutical Company for the ATHOS-3 study and through the Wake Forest Center for Hypertension and Vascular Research for RAAS in septic shock. MO’s institution received research funding from La Jolla Pharmaceutical Company. CRG and TH are employees of La Jolla Pharmaceutical Company. SK was an employee of La Jolla Pharmaceutical Company during the preparation of the manuscript.
Figures
Comment in
-
Adjunctive vasopressors in distributive shock: How soon is early?Crit Care. 2023 May 30;27(1):210. doi: 10.1186/s13054-023-04500-y. Crit Care. 2023. PMID: 37254175 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical