A chemically inducible IL-2 receptor signaling complex allows for effective in vitro and in vivo selection of engineered CD4+ T cells
- PMID: 37147803
- PMCID: PMC10421999
- DOI: 10.1016/j.ymthe.2023.04.021
A chemically inducible IL-2 receptor signaling complex allows for effective in vitro and in vivo selection of engineered CD4+ T cells
Abstract
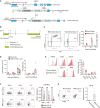
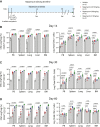
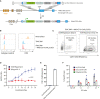
Engineered T cells represent an emerging therapeutic modality. However, complex engineering strategies can present a challenge for enriching and expanding therapeutic cells at clinical scale. In addition, lack of in vivo cytokine support can lead to poor engraftment of transferred T cells, including regulatory T cells (Treg). Here, we establish a cell-intrinsic selection system that leverages the dependency of primary T cells on IL-2 signaling. FRB-IL2RB and FKBP-IL2RG fusion proteins were identified permitting selective expansion of primary CD4+ T cells in rapamycin supplemented medium. This chemically inducible signaling complex (CISC) was subsequently incorporated into HDR donor templates designed to drive expression of the Treg master regulator FOXP3. Following editing of CD4+ T cells, CISC+ engineered Treg (CISC EngTreg) were selectively expanded using rapamycin and maintained Treg activity. Following transfer into immunodeficient mice treated with rapamycin, CISC EngTreg exhibited sustained engraftment in the absence of IL-2. Furthermore, in vivo CISC engagement increased the therapeutic activity of CISC EngTreg. Finally, an editing strategy targeting the TRAC locus permitted generation and selective enrichment of CISC+ functional CD19-CAR-T cells. Together, CISC provides a robust platform to achieve both in vitro enrichment and in vivo engraftment and activation, features likely beneficial across multiple gene-edited T cell applications.
Keywords: CAR-T; CISC; CRISPR; IL-2; T(reg); mTOR.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.J.R. is a scientific co-founder and Scientific Advisory Board (SAB) member of GentiBio, Inc., and scientific co-founder and SAB member of BeBiopharma, Inc. A.M.S. is a scientific co-founder and SAB member of GentiBio, Inc., and scientific co-founder and CEO of Umoja Biopharma. D.J.R. received past and current funding from GentiBio, Inc., for related work. D.J.R., A.M.S., P.J.C., and K.S. are inventors on patents describing methods for generating antigen-specific engineered regulatory T cells and/or use of the CISC platform. G.I.U. was a previous employee of Casebia Therapeutics and a current employee of GentiBio, Inc.
Figures





Comment in
-
Bypassing the cytokine sink: IL-2-independent IL-2 receptor signaling.Mol Ther. 2023 Aug 2;31(8):2304-2306. doi: 10.1016/j.ymthe.2023.07.007. Epub 2023 Jul 22. Mol Ther. 2023. PMID: 37482059 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

