A single ascending dose study of single-stranded oligodeoxyribonucleotide RO7062931 in Chinese healthy volunteers
- PMID: 37147890
- PMCID: PMC10339693
- DOI: 10.1111/cts.13531
A single ascending dose study of single-stranded oligodeoxyribonucleotide RO7062931 in Chinese healthy volunteers
Abstract
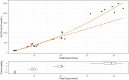
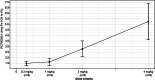
RO7062931 is an N-acetylgalactosamine (GalNAc)-conjugated single-stranded oligodeoxyribonucleotide complementary to hepatitis B virus RNA. GalNAc conjugation targets the liver through the asialoglycoprotein receptor (ASGPR). This phase I single ascending dose (SAD) study evaluated the safety, tolerability, and pharmacokinetics of RO7062931 in Chinese healthy volunteers. There were four SAD cohorts (0.3, 1.0, 2.0, and 4.0 mg/kg), in each of which healthy volunteers were randomized to a single subcutaneous (s.c.) injection of RO7062931 or matching placebo in a 4:1 ratio. Placebo recipients were pooled as one treatment group for safety assessments. A total of 41 healthy Chinese men received one dose of RO7062931 (n = 33) or placebo (n = 8) and completed the study (85-day follow-up). Adverse events (AEs) were reported in 22 of 33 (66.6%) RO7062931 recipients (n = 80 treatment-related) and seven of eight (87.5%) placebo recipients (n = 1 treatment-related). Apart from two moderate-intensity AEs, all AEs were mild. The most frequently reported AEs were influenza, injection-related reactions, and headache. Dose-proportional increases in plasma RO7062931 exposure were observed between the 0.3 and 1.0 mg/kg doses, whereas a supra-dose-proportional increase occurred at doses greater than or equal to 2.0 mg/kg, along with a marked increase in urinary excretion. Single s.c. dose of RO7062931 up to 4.0 mg/kg were safe and well-tolerated in healthy Chinese volunteers. Pharmacokinetic data suggested that ASGPR saturation had commenced between doses of 2.0 and 4.0 mg/kg. Results were broadly consistent with observations in primarily White subjects in the global first-in-human study of RO7062931.
© 2023 China Innovation Center of Roche and The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Y.Z., V.P., C.W., S.D., B.S., M.T., and J.F.G. are employees of F. Hoffman‐La Roche. All other authors declared no competing interests for this work.
Figures

References
-
- WHO . Hepatitis B. 2021. Accessed May 6, 2023. https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐b
-
- Yu R, Fan R, Hou J. Chronic hepatitis B virus infection: epidemiology, prevention, and treatment in China. Front Med. 2014;8:135‐144. - PubMed
-
- Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319:1802‐1813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

