Awareness of intratumoral bacteria and their potential application in cancer treatment
- PMID: 37148441
- PMCID: PMC10164222
- DOI: 10.1007/s12672-023-00670-x
Awareness of intratumoral bacteria and their potential application in cancer treatment
Abstract
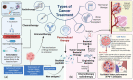
Hitherto, the recognition of the microbiota role in tumorigenesis and clinical studies mostly focused on the intestinal flora. In contrast to the gut microbiome, microorganisms resident in tumor tissue are in close contact with cancer cells and therefore have the potential to have similar or even different functional patterns to the gut flora. Some investigations have shown intratumoral bacteria, which might come from commensal microbiota in mucosal areas including the gastrointestinal tract and oral cavity, or from nearby normal tissues. The existence, origin, and interactions of intratumoral bacteria with the tumor microenvironment all contribute to intratumoral microorganism heterogeneity. Intratumoral bacteria have a significant role in tumor formation. They can contribute to cancer at the genetic level by secreting poisons that directly damage DNA and also intimately related to immune system response at the systemic level. Intratumoral bacteria have an impact on chemotherapy and immunotherapy in cancer. Importantly, various properties of bacteria such as targeting and ease of modification make them powerful candidates for precision therapy, and combining microbial therapies with other therapies is expected to improve the effectiveness of cancer treatment. In this review, we mainly described the heterogeneity and potential sources of intratumoral bacteria, overviewed the important mechanisms by which they were involved in tumor progression, and summarized their potential value in oncology therapy. At last, we highlight the problems of research in this field, and look forward to a new wave of studies using the various applications of intratumoral microorganisms in cancer therapy.
Keywords: Intratumoral bacteria; Microbial community heterogeneity; Microbiome; Omics technology; cancer treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
-
- Sadrekarimi H, Gardanova ZR, Bakhshesh M, Ebrahimzadeh F, Yaseri AF, Thangavelu L, Hasanpoor Z, Zadeh FA, Kahrizi MS. Emerging role of human microbiome in cancer development and response to therapy: special focus on intestinal microflora. J Transl Med. 2022;20:301. doi: 10.1186/s12967-022-03492-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
