Characteristics and research status among clinical trials in cardio-oncology by bibliometric and visualized analysis
- PMID: 37148538
- PMCID: PMC10278509
- DOI: 10.1002/cam4.6045
Characteristics and research status among clinical trials in cardio-oncology by bibliometric and visualized analysis
Abstract
Background: We aim to establish the characteristics of published cardio-oncology research of clinical trials by bibliometric analysis and to talk about the prospects and difficulties facing the development of cardio-oncology.
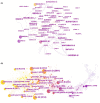
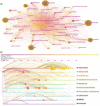
Methods: Search of data related to clinical trials in cardiac oncology from 1990 to 2022 from the Web of Science core collection. Using CiteSpace to perform co-citation analysis of authors, countries (regions) and institutions, journals and cited journals, cited authors and cited literature, and keywords.
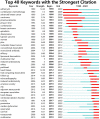
Results: Of the 607 clinical trial studies, the number of papers published per year has increased over time. The regions with the greatest influence were North America (especially the United States) and Europe. Multicenter research has always been the focus of cardio-oncology research, but cross-regional cooperation was still lacking. Myocardial toxicity caused by anthracyclines has received the earliest attention and has been studied for the longest time. Meanwhile, the efficacy and cardiotoxicity of new anticancer drugs always came into focus, but at a slow pace. Few studies on myocardial toxicity were related to the treatment of tumors except breast cancer. Risk factors, heart disease, adverse outcomes, follow-up, and intervention protection were the major hotspots revealed by co-citation cluster.
Conclusions: There is great potential for the development of clinical trials in cardio-oncology, especially in multicenter cooperation across different regions. Expansion of tumor types, myocardial toxicity of different drugs, and effective interventions in the research direction and design of clinical trials are necessary.
Keywords: CiteSpace; bibliometrics; cardiotoxicity; clinical trials; oncology; visual analysis.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflicts of interest.
Figures


Similar articles
-
Prevention and treatment of anthracycline-induced cardiotoxicity: A bibliometric analysis of the years 2000-2023.Heliyon. 2024 Apr 21;10(9):e29926. doi: 10.1016/j.heliyon.2024.e29926. eCollection 2024 May 15. Heliyon. 2024. PMID: 38698971 Free PMC article.
-
Research Trends in the Application of Artificial Intelligence in Oncology: A Bibliometric and Network Visualization Study.Front Biosci (Landmark Ed). 2022 Aug 31;27(9):254. doi: 10.31083/j.fbl2709254. Front Biosci (Landmark Ed). 2022. PMID: 36224012
-
Trends in high-impact papers in nursing research published from 2008 to 2018: A web of science-based bibliometric analysis.J Nurs Manag. 2020 Jul;28(5):1041-1052. doi: 10.1111/jonm.13038. Epub 2020 Jun 3. J Nurs Manag. 2020. PMID: 32458514
-
Opportunities and Challenges in Cardio-Oncology: A Bibliometric Analysis From 2010 to 2022.Curr Probl Cardiol. 2023 Aug;48(8):101227. doi: 10.1016/j.cpcardiol.2022.101227. Epub 2022 Apr 29. Curr Probl Cardiol. 2023. PMID: 35500730 Review.
-
Top 100 most-cited papers in core dental public health journals: bibliometric analysis.Community Dent Oral Epidemiol. 2021 Feb;49(1):40-46. doi: 10.1111/cdoe.12572. Epub 2020 Sep 15. Community Dent Oral Epidemiol. 2021. PMID: 32935344 Review.
Cited by
-
The Application of Artificial Intelligence in Thyroid Nodules: A Systematic Review Based on Bibliometric Analysis.Endocr Metab Immune Disord Drug Targets. 2024;24(11):1280-1290. doi: 10.2174/0118715303264254231117113456. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38178659
-
Bibliometric analysis of research on manual therapy for low back pain from 2013 to 2023.Medicine (Baltimore). 2025 Feb 21;104(8):e41618. doi: 10.1097/MD.0000000000041618. Medicine (Baltimore). 2025. PMID: 39993079 Free PMC article.
-
The interplay between cancer and cardiovascular disease.Hypertens Res. 2025 Mar;48(3):1192-1194. doi: 10.1038/s41440-024-02015-9. Epub 2024 Nov 14. Hypertens Res. 2025. PMID: 39543431
-
Selenium: 48-year journey of global clinical trials.Mol Cell Biochem. 2025 Jun;480(6):3253-3265. doi: 10.1007/s11010-024-05202-x. Epub 2025 Jan 4. Mol Cell Biochem. 2025. PMID: 39755855 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

