Determinants of humoral and cellular immune responses to three doses of mRNA SARS-CoV-2 vaccines in older adults: a longitudinal cohort study
- PMID: 37148891
- PMCID: PMC10156136
- DOI: 10.1016/S2666-7568(23)00055-7
Determinants of humoral and cellular immune responses to three doses of mRNA SARS-CoV-2 vaccines in older adults: a longitudinal cohort study
Abstract
Background: Older age is associated with poorer outcomes to COVID-19 infection. The Norwegian Institute of Public Health established a longitudinal cohort of adults aged 65-80 years to study the effects of the COVID-19 pandemic. Here we describe the characteristics of the cohort in general, and specifically the immune responses at baseline and after primary and booster vaccination in a subset of longitudinal blood samples, and the epidemiological factors affecting these responses.
Methods: 4551 participants were recruited, with humoral (n=299) and cellular (n=90) responses measured before vaccination and after two and three vaccine doses. Information on general health, infections, and vaccinations were obtained from questionnaires and national health registries.
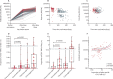
Findings: Half of the participants had a chronic condition. 849 (18·7%) of 4551 were prefrail and 184 (4%) of 4551 were frail. 483 (10·6%) of 4551 had general activity limitations (scored with the Global Activity Limitation Index). After dose two, 295 (98·7%) of 299 participants were seropositive for anti-receptor binding domain IgG, and 210 (100%) of 210 participants after dose three. Spike-specific CD4 and CD8 T cell responses showed high heterogeneity after vaccination and responded to the alpha (B.1.1.7), delta (B.1.617.2), and omicron (B.1.1.529 or BA.1) variants of concern. Cellular responses to seasonal coronaviruses increased after SARS-CoV-2 vaccination. Heterologous prime boosting with mRNA vaccines was associated with the highest antibody (p=0·019) and CD4 T cell responses (p=0·003), and hypertension with lower antibody levels after three doses (p=0·04).
Interpretation: Most older adults, including those with comorbidities, generated good serological and cellular responses after two vaccine doses. Responses further improved after three doses, particularly after heterologous boosting. Vaccination also generated cross-reactive T cells against variants of concern and seasonal coronaviruses. Frailty was not associated with impaired immune responses, but hypertension might indicate reduced responsiveness to vaccines even after three doses. Individual differences identified through longitudinal sampling enables better prediction of the variability of vaccine responses, which can help guide future policy on the need for subsequent doses and their timing.
Funding: Norwegian Institute of Public Health, Norwegian Ministry of Health, Research Council of Norway, and Coalition for Epidemic Preparedness Innovations.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

Comment in
-
The immune response to SARS-CoV-2 vaccination in older people.Lancet Healthy Longev. 2023 May;4(5):e177-e178. doi: 10.1016/S2666-7568(23)00060-0. Lancet Healthy Longev. 2023. PMID: 37148886 No abstract available.
References
-
- WHO Ageing and health. Oct 1, 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
-
- Centers for Disease Control and Prevention COVID-19 risks and vaccine information for older adults. August 4, 2021. https://stacks.cdc.gov/view/cdc/119720
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

