Longitudinal humoral and cell-mediated immune responses in a population-based cohort in Zurich, Switzerland between March and June 2022 - evidence for protection against Omicron SARS-CoV-2 infection by neutralizing antibodies and spike-specific T-cell responses
- PMID: 37149211
- PMCID: PMC10159929
- DOI: 10.1016/j.ijid.2023.04.407
Longitudinal humoral and cell-mediated immune responses in a population-based cohort in Zurich, Switzerland between March and June 2022 - evidence for protection against Omicron SARS-CoV-2 infection by neutralizing antibodies and spike-specific T-cell responses
Abstract
Objectives: The correlate(s) of protection against SARS-CoV-2 remain incompletely defined. Additional information regarding the combinations of antibody and T cell-mediated immunity which can protect against (re)infection is needed.
Methods: We conducted a population-based, longitudinal cohort study including 1044 individuals of varying SARS-CoV-2 vaccination and infection statuses. We assessed spike (S)- and nucleocapsid (N)-immunoglobulin(Ig)G and wildtype, Delta, and Omicron-neutralizing antibody (N-Ab) activity. In a subset of 328 individuals, we evaluated S, membrane (M), and N-specific T cells. Three months later, we reassessed Ab (n = 964) and T cell (n = 141) responses and evaluated factors associated with protection from (re)infection.
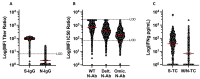
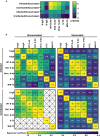
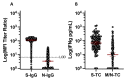
Results: At the study start, >98% of participants were S-IgG seropositive. N-IgG and M/N-T-cell responses increased over time, indicating viral (re)exposure, despite existing S-IgG. Compared to N-IgG, M/N-T cells were a more sensitive measure of viral exposure. High N-IgG titers, Omicron-N-Ab activity, and S-specific-T-cell responses were all associated with a reduced likelihood of (re)infection over time.
Conclusion: Population-level SARS-CoV-2 immunity is S-IgG-dominated, but heterogeneous. M/N-T-cell responses can distinguish previous infection from vaccination, and monitoring a combination of N-IgG, Omicron-N-Ab, and S-T-cell responses may help estimate protection against SARS-CoV-2 (re)infection.
Keywords: Hybrid immunity; Interferon-gamma release assay; Neutralizing antibodies; SARS-CoV-2; Seroprevalence; T cell responses.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declarations of competing interest The authors have no competing interests to declare.
Figures


Similar articles
-
Robust Vaccine-Induced as Well as Hybrid B- and T-Cell Immunity across SARS-CoV-2 Vaccine Platforms in People with HIV.Microbiol Spectr. 2023 Jun 15;11(3):e0115523. doi: 10.1128/spectrum.01155-23. Epub 2023 May 11. Microbiol Spectr. 2023. PMID: 37166335 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study.Lancet Microbe. 2022 May;3(5):e348-e356. doi: 10.1016/S2666-5247(22)00036-2. Epub 2022 Mar 23. Lancet Microbe. 2022. PMID: 35345417 Free PMC article.
-
The potential clinical utility of measuring severe acute respiratory syndrome coronavirus 2-specific T-cell responses.Clin Microbiol Infect. 2021 Dec;27(12):1784-1789. doi: 10.1016/j.cmi.2021.07.005. Epub 2021 Jul 10. Clin Microbiol Infect. 2021. PMID: 34256141 Free PMC article. Review.
-
Major Update 2: Antibody Response and Risk for Reinfection After SARS-CoV-2 Infection-Final Update of a Living, Rapid Review.Ann Intern Med. 2023 Jan;176(1):85-91. doi: 10.7326/M22-1745. Epub 2022 Nov 29. Ann Intern Med. 2023. PMID: 36442059 Free PMC article. Review.
Cited by
-
Prevalence of SARS-CoV-2-specific antibodies in a sample of the Lithuanian population-based study in Spring 2023.Heliyon. 2024 Apr 12;10(8):e29343. doi: 10.1016/j.heliyon.2024.e29343. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38681561 Free PMC article.
-
Meta-analysis of hybrid immunity to mitigate the risk of Omicron variant reinfection.Front Public Health. 2024 Aug 26;12:1457266. doi: 10.3389/fpubh.2024.1457266. eCollection 2024. Front Public Health. 2024. PMID: 39253287 Free PMC article.
-
Cellular Immunity of SARS-CoV-2 in the Borriana COVID-19 Cohort: A Nested Case-Control Study.Epidemiologia (Basel). 2024 Apr 10;5(2):167-186. doi: 10.3390/epidemiologia5020012. Epidemiologia (Basel). 2024. PMID: 38651389 Free PMC article.
-
Monitoring of antibodies against SARS-CoV-2 over 2 years and characterization of immune responses following Omicron infection in a South Indian community cohort.Sci Rep. 2025 Jul 9;15(1):24756. doi: 10.1038/s41598-025-10447-7. Sci Rep. 2025. PMID: 40634661 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

