Cost-effectiveness of point-of-care versus centralised, laboratory-based nucleic acid testing for diagnosis of HIV in infants: a systematic review of modelling studies
- PMID: 37149292
- PMCID: PMC10175481
- DOI: 10.1016/S2352-3018(23)00029-2
Cost-effectiveness of point-of-care versus centralised, laboratory-based nucleic acid testing for diagnosis of HIV in infants: a systematic review of modelling studies
Abstract
Background: Point-of-care (POC) nucleic acid testing for diagnosis of HIV in infants facilitates earlier initiation of antiretroviral therapy (ART) than with centralised (standard-of-care, SOC) testing, but can be more expensive. We evaluated cost-effectiveness data from mathematical models comparing POC with SOC to provide global policy guidance.
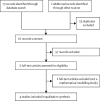
Methods: In this systematic review of modelling studies, we searched PubMed, MEDLINE, Embase, the National Health Service Economic Evaluation Database, Econlit, and conference abstracts, combining terms for "HIV" + "infant"/"early infant diagnosis" + "point-of-care" + "cost-effectiveness" + "mathematical models", without restrictions from database inception to July 15, 2022. We selected reports of mathematical cost-effectiveness models comparing POC with SOC for HIV diagnosis in infants younger than 18 months. Titles and abstracts were independently reviewed, with full-text review for qualifying articles. We extracted data on health and economic outcomes and incremental cost-effectiveness ratios (ICERs) for narrative synthesis. The primary outcomes of interest were ICERs (comparing POC with SOC) for ART initiation and survival of children living with HIV.
Findings: Our search identified 75 records through database search. 13 duplicates were excluded, leaving 62 non-duplicate articles. 57 records were excluded and five were reviewed in full text. One article was excluded as it was not a modelling study, and four qualifying studies were included in the review. These four reports were from two mathematical models from two independent modelling groups. Two reports used the Johns Hopkins model to compare POC with SOC for repeat early infant diagnosis testing in the first 6 months in sub-Saharan Africa (first report, simulation of 25 000 children) and Zambia (second report, simulation of 7500 children). In the base scenario, POC versus SOC increased probability of ART initiation within 60 days of testing from 19% to 82% (ICER per additional ART initiation range US$430-1097; 9-month cost horizon) in the first report; and from 28% to 81% in the second ($23-1609, 5-year cost horizon). Two reports compared POC with SOC for testing at 6 weeks in Zimbabwe using the Cost-Effectiveness of Preventing AIDS Complications-Paediatric model (simulation of 30 million children; lifetime horizon). POC increased life expectancy and was considered cost-effective compared with SOC (ICER $711-850 per year of life saved in HIV-exposed children). Results were robust throughout sensitivity and scenario analyses. In most scenarios, platform cost-sharing (co-use with other programmes) resulted in POC being cost-saving compared with SOC.
Interpretation: Four reports from two different models suggest that POC is a cost-effective and potentially cost-saving strategy for upscaling of early infant testing compared with SOC.
Funding: Bill & Melinda Gates Foundation, Unitaid, National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, WHO, and Massachusetts General Hospital Research Scholars.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Comment in
-
Economics of point-of-care infant HIV tests.Lancet HIV. 2023 May;10(5):e278-e279. doi: 10.1016/S2352-3018(23)00053-X. Lancet HIV. 2023. PMID: 37149290 No abstract available.
References
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. - PubMed
-
- Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. - PubMed
-
- WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. https://www.who.int/publications/i/item/9789240031593 - PubMed
-
- UNICEF Key considerations for introducing new HIV point-of-care diagnostic technologies in national health systems. 2018. http://childrenandaids.org/sites/default/files/poc-toolkit/KCD_draft_Eng...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical