Efficacy of primary series AZD1222 (ChAdOx1 nCoV-19) vaccination against SARS-CoV-2 variants of concern: Final analysis of a randomized, placebo-controlled, phase 1b/2 study in South African adults (COV005)
- PMID: 37149443
- PMCID: PMC10133888
- DOI: 10.1016/j.vaccine.2023.04.058
Efficacy of primary series AZD1222 (ChAdOx1 nCoV-19) vaccination against SARS-CoV-2 variants of concern: Final analysis of a randomized, placebo-controlled, phase 1b/2 study in South African adults (COV005)
Abstract
COVID-19 vaccine efficacy (VE) has been observed to vary against antigenically distinct SARS-CoV-2 variants of concern (VoC). Here we report the final analysis of VE and safety from COV005: a phase 1b/2, multicenter, double-blind, randomized, placebo-controlled study of primary series AZD1222 (ChAdOx1 nCoV-19) vaccination in South African adults aged 18-65 years. South Africa's first, second, and third waves of SARS-CoV-2 infections were respectively driven by the ancestral SARS-CoV-2 virus (wild type, WT), and SARS-CoV-2 Beta and Delta VoCs. VE against asymptomatic and symptomatic infection was 90.6% for WT, 6.7% for Beta and 77.1% for Delta. No cases of severe COVID-19 were documented ahead of unblinding. Safety was consistent with the interim analysis, with no new safety concerns identified. Notably, South Africa's Delta wave occurred ≥ 9 months after primary series vaccination, suggesting that primary series AZD1222 vaccination offers a good durability of protection, potentially due to an anamnestic response. Clinical trial identifier: CT.gov NCT04444674.
Keywords: AZD1222; COVID-19; ChAdOx1 nCov-19; SARS-CoV-2 variant; Vaccine efficacy.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Shabir Madhi reports financial support was provided by GlaxoSmithKline. Shabir Madhi reports financial support was provided by AstraZeneca. Shabir Madhi reports financial support was provided by BMGF. Shabir Madhi reports financial support was provided by South African Medical Research Council. Shabir Madhi reports financial support was provided by Pfizer. Shabir Madhi reports financial support was provided by Minervax. Shabir Madhi reports financial support was provided by Novavax. Shabir Madhi reports financial support was provided by Providence. Shabir Madhi reports financial support was provided by Greenstone. Shabir Madhi reports financial support was provided by ImmunityBio. Clare L. Cutland reports financial support was provided by BMGF. Clare L. Cutland reports financial support was provided by Pfizer. Clare L. Cutland reports financial support was provided by Biovac. Lee Fairlie reports financial support was provided by South African Medical Research Council. Lee Fairlie reports financial support was provided by Bill and Melinda Gates Foundation. Mookho Malahleha reports financial support was provided by Synergy Biomed Research Institute. Andrew J. Pollard reports financial support was provided by University of Oxford. Andrew J. Pollard reports financial support was provided by Shionogi. Andrew J. Pollard reports financial support was provided by AstraZeneca. Andrew J. Pollard reports financial support was provided by National Institute for Health and Care Research. Qasim Ebrahim Bhorat reports financial support was provided by Wits Health Consortium. Qasim Ebrahim Bhorat reports financial support was provided by Regeneron Pharmaceuticals. Qasim Ebrahim Bhorat reports financial support was provided by GlaxoSmithKline. Qasim Ebrahim Bhorat reports financial support was provided by Avillion. Qasim Ebrahim Bhorat reports financial support was provided by Sanofi. Qasim Ebrahim Bhorat reports financial support was provided by NovoNordisk. Qasim Ebrahim Bhorat reports financial support was provided by Bill & Melinda Gates Foundation. Qasim Ebrahim Bhorat reports financial support was provided by South African Medical Research Council. Qasim Ebrahim Bhorat reports financial support was provided by Novavax. Qasim Ebrahim Bhorat reports financial support was provided by AstraZeneca. Qasim Ebrahim Bhorat reports financial support was provided by Clover BioPharmaceticals. Tonya L. Villafana reports financial support was provided by AstraZeneca. Clare L. Cutland reports a relationship with BMGF that includes: employment and funding grants. Mookho Malahleha reports a relationship with RTI that includes: consulting or advisory. Andrew J. Pollard reports a relationship with UK Joint Committee on Vaccines and Immunization that includes: board membership. Andrew J. Pollard reports a relationship with World Health Organisation SAGE that includes: board membership. Sarah Gilbert reports a relationship with Vaccitech that includes: equity or stocks. Teresa Lambe reports a relationship with Vaccitech that includes: consulting or advisory. Mookho Malahleha reports a relationship with Synergy Biomed Research Institute that includes: board membership, employment, and equity or stocks. Sarah Gilbert has patent issued to Vaccitech. Teresa Lambe has patent issued to Vaccitech.
Figures
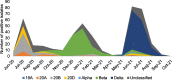

References
-
- Network for Genomic Surveillance South Africa (NGS-SA). SARS-CoV-2 Sequencing Update 12 November 2021. Available at: https://www.nicd.ac.za/wp-content/uploads/2021/11/Update-of-SA-sequencin.... Accessed 22 April 2022.
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

