Influence of the ligand-field on EPR parameters of cis- and trans-isomers in MoV systems relevant to molybdenum enzymes: Experimental and density functional theory study
- PMID: 37149488
- PMCID: PMC10330323
- DOI: 10.1016/j.jinorgbio.2023.112228
Influence of the ligand-field on EPR parameters of cis- and trans-isomers in MoV systems relevant to molybdenum enzymes: Experimental and density functional theory study
Abstract
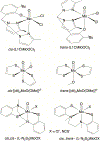
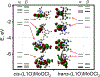
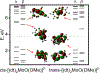
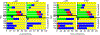
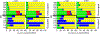
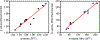
The electron paramagnetic resonance (EPR) investigation of mononuclear cis- and trans-(L1O)MoOCl2 complexes [L1OH = bis(3,5-dimethylpyrazolyl)-3-tert-butyl-2-hydroxy-5-methylphenyl)methane] reveals a significant difference in their spin Hamiltonian parameters which reflect different equatorial and axial ligand fields created by the heteroscorpionate donor atoms. Density functional theory (DFT) was used to calculate the values of principal components and relative orientations of the g and A tensors, and the molecular framework in four pairs of isomeric mononuclear oxo‑molybdenum(V) complexes (cis- and trans-(L1O)MoOCl2, cis,cis- and cis,trans-(L-N2S2)MoOCl [L-N2S2H2 = N,N'-dimethyl-N,N'-bis(mercaptophenyl)ethylenediamine], cis,cis- and cis,trans-(L-N2S2)MoO(SCN), and cis- and trans-[(dt)2MoO(OMe)]2- [dtH2 = 2,3-dimercapto-2-butene]). Scalar relativistic DFT calculations were conducted using three different exchange-correlation functionals. It was found that the use of hybrid exchange-correlation functional with 25% of the Hartree-Fock exchange leads to the best quantitative agreement between theory and experiment. A simplified ligand-field approach was used to analyze the influence of the ligand fields in all cis- and trans-isomers on energies and contributions of molybdenum d-orbital manifold to g and A tensors and relative orientations. Specifically, contributions that originated from the spin-orbit coupling of the dxz, dyz, and dx2-y2 orbitals into the ground state have been discussed. The new findings are discussed in the context of the experimental data of mononuclear molybdoenzyme, DMSO reductase.
Keywords: DFT calculations; DMSO reductase; EPR spectroscopy; Molybdenum enzymes; Molybdenum hyperfine parameters.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
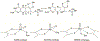

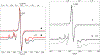


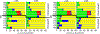
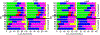
References
-
- Metzger MC, Basu P, Pterin-Containing Microbial Molybdenum Enzymes, in: Microbial Metabolism of Metals and Metalloids, Springer, 2022, pp. 359–415.
-
- Kisker C, Schindelin H, Rees DC, Annual review of biochemistry, 66 (1997) 233–267. - PubMed
-
- Mintmier B, McGarry JM, Bain DJ, Basu P, JBIC J Biol. Inorg. Chem, 26 (2021) 13–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

