Model-based assessment of combination therapies - ranking of radiosensitizing agents in oncology
- PMID: 37149596
- PMCID: PMC10164338
- DOI: 10.1186/s12885-023-10899-y
Model-based assessment of combination therapies - ranking of radiosensitizing agents in oncology
Abstract
Background: To increase the chances of finding efficacious anticancer drugs, improve development times and reduce costs, it is of interest to rank test compounds based on their potential for human use as early as possible in the drug development process. In this paper, we present a method for ranking radiosensitizers using preclinical data.
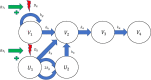
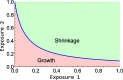
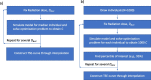
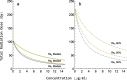
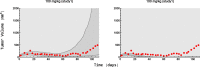
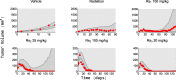
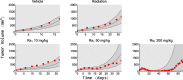
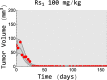
Methods: We used data from three xenograft mice studies to calibrate a model that accounts for radiation treatment combined with radiosensitizers. A nonlinear mixed effects approach was utilized where between-subject variability and inter-study variability were considered. Using the calibrated model, we ranked three different Ataxia telangiectasia-mutated inhibitors in terms of anticancer activity. The ranking was based on the Tumor Static Exposure (TSE) concept and primarily illustrated through TSE-curves.
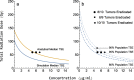
Results: The model described data well and the predicted number of eradicated tumors was in good agreement with experimental data. The efficacy of the radiosensitizers was evaluated for the median individual and the 95% population percentile. Simulations predicted that a total dose of 220 Gy (5 radiation sessions a week for 6 weeks) was required for 95% of tumors to be eradicated when radiation was given alone. When radiation was combined with doses that achieved at least 8 [Formula: see text] of each radiosensitizer in mouse blood, it was predicted that the radiation dose could be decreased to 50, 65, and 100 Gy, respectively, while maintaining 95% eradication.
Conclusions: A simulation-based method for calculating TSE-curves was developed, which provides more accurate predictions of tumor eradication than earlier, analytically derived, TSE-curves. The tool we present can potentially be used for radiosensitizer selection before proceeding to subsequent phases of the drug discovery and development process.
Keywords: Combination therapy; Inter-study variability; Non-linear mixed effects; Radiation therapy; Tumor static exposure.
© 2023. The Author(s).
Conflict of interest statement
FL is an employee of Merck Healthcare KGaA, Darmstadt, Germany.
AZ is an employee of Merck Healthcare KGaA, Darmstadt, Germany.
SB was an employee of Merck Healthcare KGaA, Darmstadt, Germany, at the time of the study.
Figures


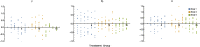

References
-
- Philips T, Hoppe R, Roach M. Leibel and Phillips Textbook of Radiation Oncology. 3rd ed. 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

