Transmission risk of Oropouche fever across the Americas
- PMID: 37149619
- PMCID: PMC10163756
- DOI: 10.1186/s40249-023-01091-2
Transmission risk of Oropouche fever across the Americas
Abstract
Background: Vector-borne diseases (VBDs) are important contributors to the global burden of infectious diseases due to their epidemic potential, which can result in significant population and economic impacts. Oropouche fever, caused by Oropouche virus (OROV), is an understudied zoonotic VBD febrile illness reported in Central and South America. The epidemic potential and areas of likely OROV spread remain unexplored, limiting capacities to improve epidemiological surveillance.
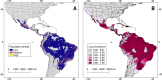
Methods: To better understand the capacity for spread of OROV, we developed spatial epidemiology models using human outbreaks as OROV transmission-locality data, coupled with high-resolution satellite-derived vegetation phenology. Data were integrated using hypervolume modeling to infer likely areas of OROV transmission and emergence across the Americas.
Results: Models based on one-support vector machine hypervolumes consistently predicted risk areas for OROV transmission across the tropics of Latin America despite the inclusion of different parameters such as different study areas and environmental predictors. Models estimate that up to 5 million people are at risk of exposure to OROV. Nevertheless, the limited epidemiological data available generates uncertainty in projections. For example, some outbreaks have occurred under climatic conditions outside those where most transmission events occur. The distribution models also revealed that landscape variation, expressed as vegetation loss, is linked to OROV outbreaks.
Conclusions: Hotspots of OROV transmission risk were detected along the tropics of South America. Vegetation loss might be a driver of Oropouche fever emergence. Modeling based on hypervolumes in spatial epidemiology might be considered an exploratory tool for analyzing data-limited emerging infectious diseases for which little understanding exists on their sylvatic cycles. OROV transmission risk maps can be used to improve surveillance, investigate OROV ecology and epidemiology, and inform early detection.
Keywords: Convex-hulls; Distribution modeling; Hypervolumes; One-class support vector machines; Oropouche fever; Oropouche virus; Risk mapping; Spatial modeling.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Durango-Chavez HV, Toro-Huamanchumo CJ, Silva-Caso W, Martins-Luna J, Aguilar-Luis MA, del Valle-Mendoza J, et al. Oropouche virus infection in patients with acute febrile syndrome: is a predictive model based solely on signs and symptoms useful? PLoS ONE. 2022;17:e0270294. doi: 10.1371/journal.pone.0270294. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

