Germline drivers of gynecologic carcinosarcomas
- PMID: 37149903
- PMCID: PMC10330315
- DOI: 10.1016/j.ygyno.2023.04.024
Germline drivers of gynecologic carcinosarcomas
Abstract
Objectives: To describe the prevalence of germline pathogenic variants (gPVs) in endometrial and ovarian carcinosarcomas and determine if gPVs are drivers of carcinosarcoma.
Methods: Patients with endometrial or ovarian carcinosarcomas who underwent clinical tumor-normal sequencing from 1/1/2015 to 6/1/2021 and consented to germline assessment of ≥76 cancer predisposition genes were included. In patients with gPVs, biallelic inactivation was identified through analysis of loss of heterozygosity and somatic pathogenic alterations.
Results: Of 216 patients identified, 167 (77%) were diagnosed with endometrial carcinosarcoma and 49 (23%) with ovarian carcinosarcoma. Overall, 33 gPVs were observed in 29 patients (13%); 20 gPVs (61%) had biallelic loss in tumors. The rate of high-penetrance gPVs overall was 7% (16 of 216); 88% of high-penetrance gPVs had biallelic loss. In the endometrial carcinosarcoma cohort, 22 gPVs were found in 19 (11%) of 167 patients; 12 gPVs (55%) had biallelic loss in tumors, including 8 (89%) of 9 in high-penetrance gPVs. Among the ovarian carcinosarcoma cohort, 11 gPVs were found in 10 (20%) of 49 patients; 8 gPVs (73%) had biallelic loss in tumors, and all evaluable high-penetrance gPVs (n = 6) had biallelic loss. All gPVs in homologous recombination (BRCA1, BRCA2, RAD51C) and Lynch syndrome (MSH2, MSH6) genes had biallelic loss in tumors (n = 15).
Conclusions: gPVs in genes affecting homologous recombination- or Lynch-associated mismatch repair exhibited biallelic inactivation within tumors, suggesting likely drivers of gynecologic carcinosarcoma. Our data support germline testing for patients with gynecologic carcinosarcomas, given implications for treatment and risk-reduction in patients and at-risk family members.
Keywords: Endometrial carcinosarcoma; Genetic testing; Germline pathogenic variants; Gynecologic carcinosarcomas; Ovarian carcinosarcoma.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest B.Weigelt reports research funding and scientific advisory board participation from Repare Therapeutics, outside the submitted work. K. Offit is a founder and shareholder of AnaNeo Therapeutics Incorporated. Z.K. Stadler has an immediate family member who serves as a consultant in Ophthalmology for Alcon, Adverum, Gyroscope Therapeutics Ltd., Neurogene and RegenexBio, outside the submitted work. M.F. Berger reports receiving research funding from GRAIL and advisory board activities for Eli Lilly, AstraZeneca, and PetDx. Y.L. Liu reports research funding from AstraZeneca, GSK, and Repare Therapeutics. N.R. Abu-Rustum reports research funding paid to the institution from GRAIL. C. Aghajanian reports clinical trial funding paid to the institution from AstraZeneca; consulting fees (advisory board) from Eisai/Merck, Roche/Genentech, Abbvie, AstraZeneca/Merck, and Repare Therapeutics; advisory board participation (no fee) for Blueprint Medicine; and leadership/fiduciary roles for the GOG Foundation Board of Directors (travel cost reimbursement) and NRG Oncology Board of Directors (unpaid). D.S. Chi reports personal fees from Apyx Medical, Verthermia Inc., Biom ‘Up, and AstraZeneca, as well as recent or current stock/options ownership of Apyx Medical, Verthemia, Intuitive Surgical, Inc., TransEnterix, Inc., Doximity, Moderna, and BioNTech SE. R.N. Grisham reports personal fees from AstraZeneca, Corcept, GlaxoSmithKline, MJH Life Sciences, Natera, PER, and SpringWorks. M.M. Leitao Jr. reports research funding paid to the institution from KCI/Acelity, ad-hoc speaker for Intuitive Surgical, Inc., and advisory board participation for JnJ/Ethicon and Takeda. V. Makker reports advisory board participation (unpaid) for Eisai, Merck, Clovis, Faeth, Duality, Morphyes, Karyopharm, Novartis, Lilly, and Immunocore. R.E. O'Cearbhaill reports honoraria from GSK, Bayer, Regeneron, SeaGen, Fresenius Kabi, Immunogen, MJH Life Sciences, Curio, R-Pharm, GOG Foundation, and Onclive/PER. M. Rubinstein reports research funding from Merk, Zentalis, and AstraZeneca. M.L. Hensley reports advisory board participation at Aadi Bioscience, GSK, Thrive Bioscience, and Lilly; has an immediate family member who is employed by Sanofi; and served as a CME faculty speaker for Research to Practice. All other authors have no potential conflicts of interest to disclose.
Figures
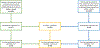
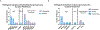
References
-
- Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: A review of the literature. Gynecol Oncol. 2015;137(3):581–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

