Morphological and biomechanical analyses of the human healthy and glaucomatous aqueous outflow pathway: Imaging-to-modeling
- PMID: 37149973
- PMCID: PMC11753070
- DOI: 10.1016/j.cmpb.2023.107485
Morphological and biomechanical analyses of the human healthy and glaucomatous aqueous outflow pathway: Imaging-to-modeling
Abstract
Background and objective: Intraocular pressure (IOP) is maintained via a dynamic balance between the production of aqueous humor and its drainage through the trabecular meshwork (TM), juxtacanalicular connective tissue (JCT), and Schlemm's canal (SC) endothelium of the conventional outflow pathway. Primary open angle glaucoma (POAG) is often associated with IOP elevation that occurs due to an abnormally high outflow resistance across the outflow pathway. Outflow tissues are viscoelastic and actively interact with aqueous humor dynamics through a two-way fluid-structure interaction coupling. While glaucoma affects the morphology and stiffness of the outflow tissues, their biomechanics and hydrodynamics in glaucoma eyes remain largely unknown. This research aims to develop an image-to-model method allowing the biomechanics and hydrodynamics of the conventional aqueous outflow pathway to be studied.
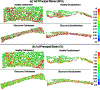
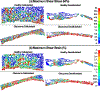
Methods: We used a combination of X-ray computed tomography and scanning electron microscopy to reconstruct high-fidelity, eye-specific, 3D microstructural finite element models of the healthy and glaucoma outflow tissues in cellularized and decellularized conditions. The viscoelastic TM/JCT/SC complex finite element models with embedded viscoelastic beam elements were subjected to a physiological IOP load boundary; the stresses/strains and the flow state were calculated using fluid-structure interaction and computational fluid dynamics.
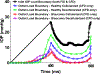
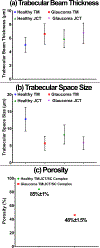
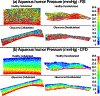
Results: Based on the resultant hydrodynamics parameters across the outflow pathway, the primary site of outflow resistance in healthy eyes was in the JCT and immediate vicinity of the SC inner wall, while the majority of the outflow resistance in the glaucoma eyes occurred in the TM. The TM and JCT in the glaucoma eyes showed 1.32-fold and 1.13-fold larger beam thickness and smaller trabecular space size (2.24-fold and 1.50-fold) compared to the healthy eyes.
Conclusions: Characterizing the accurate morphology of the outflow tissues may significantly contribute to constructing more accurate, robust, and reliable models, that can eventually help to better understand the dynamic IOP regulation, hydrodynamics of the aqueous humor, and outflow resistance dynamic in the human eyes. This model demonstrates proof of concept for determining changes to outflow resistance in healthy and glaucomatous tissues and thus may be utilized in larger cohorts of donor tissues where disease specificity, race, age, and gender of the eye donors may be accounted for.
Keywords: Computational fluid dynamics; Fluid-structure interaction; Juxtacanalicular tissue; Schlemm's canal; Trabecular meshwork.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Ellingsen BA, Grant WM, The relationship of pressure and aqueous outflow in enucleated human eyes, Invest Ophthalmol 10(6) (1971) 430–7. - PubMed
-
- Moses RA, The effect of intraocular pressure on resistance to outflow, Surv Ophthalmol 22(2) (1977) 88–100. - PubMed
-
- Johnson MC, Kamm RD, The role of Schlemm's canal in aqueous outflow from the human eye, Invest Ophthalmol Vis Sci 24(3) (1983) 320–5. - PubMed
-
- Johnson DH, Johnson M, How does nonpenetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery, J Glaucoma 10(1) (2001) 55–67. - PubMed
-
- Kitazawa Y, Horie T, Diurnal variation of intraocular pressure in primary open-angle glaucoma, Am J Ophthalmol 79(4) (1975) 557–66. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

