Rates of clinical remission and inadequate response to advanced therapies among patients with ulcerative colitis in Germany
- PMID: 37150784
- PMCID: PMC10164668
- DOI: 10.1007/s00384-023-04397-7
Rates of clinical remission and inadequate response to advanced therapies among patients with ulcerative colitis in Germany
Abstract
Purpose: Many patients treated for ulcerative colitis (UC) do not achieve clinical remission. This real-world study assessed clinical remission and inadequate response rates among patients with UC in Germany treated with advanced therapies.
Methods: This retrospective chart review included patients with UC newly initiating advanced (index) therapy (anti-TNFα agents, vedolizumab, tofacitinib) from January 2017-September 2019 (index date). Included patients had data for ≥ 12 months before (baseline period) and after the index date (follow-up period). Remission was defined as a partial Mayo score ≤ 1. Indicators of inadequate response were: index therapy discontinuation; therapy adjustments (index therapy dose escalation; augmentation with non-advanced therapies; corticosteroid [CS] use during maintenance therapy); CS dependency (use for ≥ 12 weeks); and UC-related hospitalisation, surgery or emergency department visit. Time to first remission and inadequate response were analyzed using Kaplan-Meier analyses.
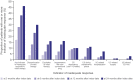
Results: Among 149 patients with UC (median age: 40 years), 96 (64.4%) were biologic-naïve and 42 (28.2%) received CS at the index date. Within 12 months, 52 patients (47.2%) were in remission; of these, 13 patients (25.0%) received ≥ 1 therapy adjustment. At 12 months, 55 patients (37.6%) had ≥ 1 indicator of an inadequate response. Median time to remission was longer among biologic-experienced vs biologic-naïve patients (24 vs 7 months; p = 0.012).
Conclusion: Over half of the patients were not in clinical remission after 12 months and more than one-third experienced inadequate response. One-quarter of patients in remission required therapy adjustments. Patients with UC require therapies that are more effective than those currently available to achieve better treatment outcomes.
Keywords: Advanced therapy; Inadequate response; Real-world treatment; Ulcerative colitis.
© 2023. The Author(s).
Conflict of interest statement
Bernd Bokemeyer received consultancy fees and speaking fees from AbbVie, Arena, Biogen, Celgene, Celltrion, Falk, Ferring, Galapagos Biopharma Deutschland GmbH, Janssen, MSD, Pfizer and Takeda. Nils Picker and Daniel Kromer are employees of Ingress-Health; the work of Ingress-Health in this study was funded by Galapagos NV. Ludger Rosin is an employee of Galapagos Biopharma Deutschland GmbH. Haridarshan Patel is a former employee of Galapagos NV.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

