Building consensus around the assessment and interpretation of Symbiodiniaceae diversity
- PMID: 37151292
- PMCID: PMC10162043
- DOI: 10.7717/peerj.15023
Building consensus around the assessment and interpretation of Symbiodiniaceae diversity
Abstract
Within microeukaryotes, genetic variation and functional variation sometimes accumulate more quickly than morphological differences. To understand the evolutionary history and ecology of such lineages, it is key to examine diversity at multiple levels of organization. In the dinoflagellate family Symbiodiniaceae, which can form endosymbioses with cnidarians (e.g., corals, octocorals, sea anemones, jellyfish), other marine invertebrates (e.g., sponges, molluscs, flatworms), and protists (e.g., foraminifera), molecular data have been used extensively over the past three decades to describe phenotypes and to make evolutionary and ecological inferences. Despite advances in Symbiodiniaceae genomics, a lack of consensus among researchers with respect to interpreting genetic data has slowed progress in the field and acted as a barrier to reconciling observations. Here, we identify key challenges regarding the assessment and interpretation of Symbiodiniaceae genetic diversity across three levels: species, populations, and communities. We summarize areas of agreement and highlight techniques and approaches that are broadly accepted. In areas where debate remains, we identify unresolved issues and discuss technologies and approaches that can help to fill knowledge gaps related to genetic and phenotypic diversity. We also discuss ways to stimulate progress, in particular by fostering a more inclusive and collaborative research community. We hope that this perspective will inspire and accelerate coral reef science by serving as a resource to those designing experiments, publishing research, and applying for funding related to Symbiodiniaceae and their symbiotic partnerships.
Keywords: Cnidarian; Collaborative; Community; Coral; Genetic diversity; ITS2; Population; Species; Symbiodiniaceae; Symbiosis.
© 2023 Davies et al.
Conflict of interest statement
Anastazia T. Banaszak and James Davis Reimer are Academic Editors for PeerJ.
Figures

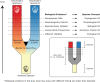

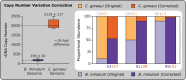

References
-
- Abrego D, Ulstrup KE, Willis BL, van Oppen MJH. Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1648):2273–2282. doi: 10.1098/rspb.2008.0180. - DOI - PMC - PubMed
-
- Adams LM, Cumbo VR, Takabayashi M. Exposure to sediment enhances primary acquisition of Symbiodinium by asymbiotic coral larvae. Marine Ecology Progress Series. 2009;377:149–156. doi: 10.3354/meps07834. - DOI
-
- Ahmadia GN, Cheng SH, Andradi-Brown DA, Baez SK, Barnes MD, Bennett NJ, Campbell SJ, Darling E, Estradivari, Gill DA, Gress E, Gurney GG, Horigue V, Jakub R, Kennedy EV, Mahajan SL, Mangubhai S, Matsuda SB, Muthiga NA, Navarro MO, Santodomingo N, Vallès H, Veverka L, Villagomez A, Wenger AS, Wosu A. Limited progress in improving gender and geographic representation in coral reef science. Frontiers in Marine Science. 2021;8:731037. doi: 10.3389/fmars.2021.731037. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

