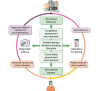
Practical approaches to building up a cardiorenal clinic
- PMID: 37151411
- PMCID: PMC10157785
- DOI: 10.1093/ckj/sfac258
Practical approaches to building up a cardiorenal clinic
Abstract
The population with concomitant heart and kidney disease (often termed 'cardiorenal' disease) is expected to grow, significantly impacting public health and healthcare utilization. Moreover, the cardiorenal nexus encompasses a bidirectional relationship that worsens prognosis and may complicate pharmacological management in often elderly and frail patients. Therefore, a more cohesive multidisciplinary team approach aiming to provide holistic, coordinated and specialized care would be a positive shift towards improving patient outcomes and optimizing healthcare resources. This article aims to define the organizational aspects and key elements for setting up a multidisciplinary cardiorenal clinical program as a potential healthcare model adapted to the particular characteristics of patients with cardiorenal disease.
Keywords: cardiorenal clinics; cardiorenal disease; cardiorenal program; heart failure; kidney disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
C.R. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Asahi, Baxter, Biomerieux, Jafron, Medica and GE and participation on data safety monitoring boards/advisory boards for Baxter, Jafron and Biomerieux. D.B. reports research grants from Kidney Research UK and AstraZeneca ESR, payment for lecture fees from AstraZeneca and Vifor Pharma and participation on an advisory board for Bayer. J.L.G. reports consulting fees from Bayer, AstraZeneca and Boehringer Ingelheim; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Bayer, AstraZeneca, Novo Nordisk and Boehringer Ingelheim and support for attending meetings and/or travel from Vifor Pharma. B.Q. reports consulting fees, participation on data safety monitoring boards or advisory boards, support for attending meetings and/or travel and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Novartis, AstraZeneca, Sandoz, Laboratorios Bial, Esteve, Sanofi-Genzyme and Otsuka and is the secretary of the Spanish Society of Nephrology. J.D. reports consulting fees and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Bayer and Vifor Pharma and consulting fees from GlaxoSmithKline. M.J.S. reports grants or contracts from Instituto Carlos III, Marató TV3 and Boehringer Ingelheim; honoraria for lectures from Novo Nordisk, Jansen, Boehringer Ingelheim, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor Pharma, ICU Medical, Fresenius and Travere Therapeutics; support for attending meetings and/or travel from Travere and participation on advisory boards for Novo Nordisk, Jansen, Boehringer Ingelheim, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor Pharma, ICU Medical, Fresenius, Travere Therapeutics and GE Healthcare. M.J.S. and J.N. are members of the CKJ editorial board. All other authors declare no competing interests.
Figures
References
-
- Savarese G, Becher PM, Lund LH et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2022;doi: 10.1093/cvr/cvac013. - PubMed
-
- McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. - PubMed
Publication types
LinkOut - more resources
Full Text Sources