From CO2 to DME: Enhancement through Heteropoly Acids from a Catalyst Screening and Stability Study
- PMID: 37151500
- PMCID: PMC10157840
- DOI: 10.1021/acsomega.3c00149
From CO2 to DME: Enhancement through Heteropoly Acids from a Catalyst Screening and Stability Study
Abstract
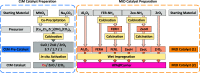
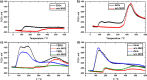
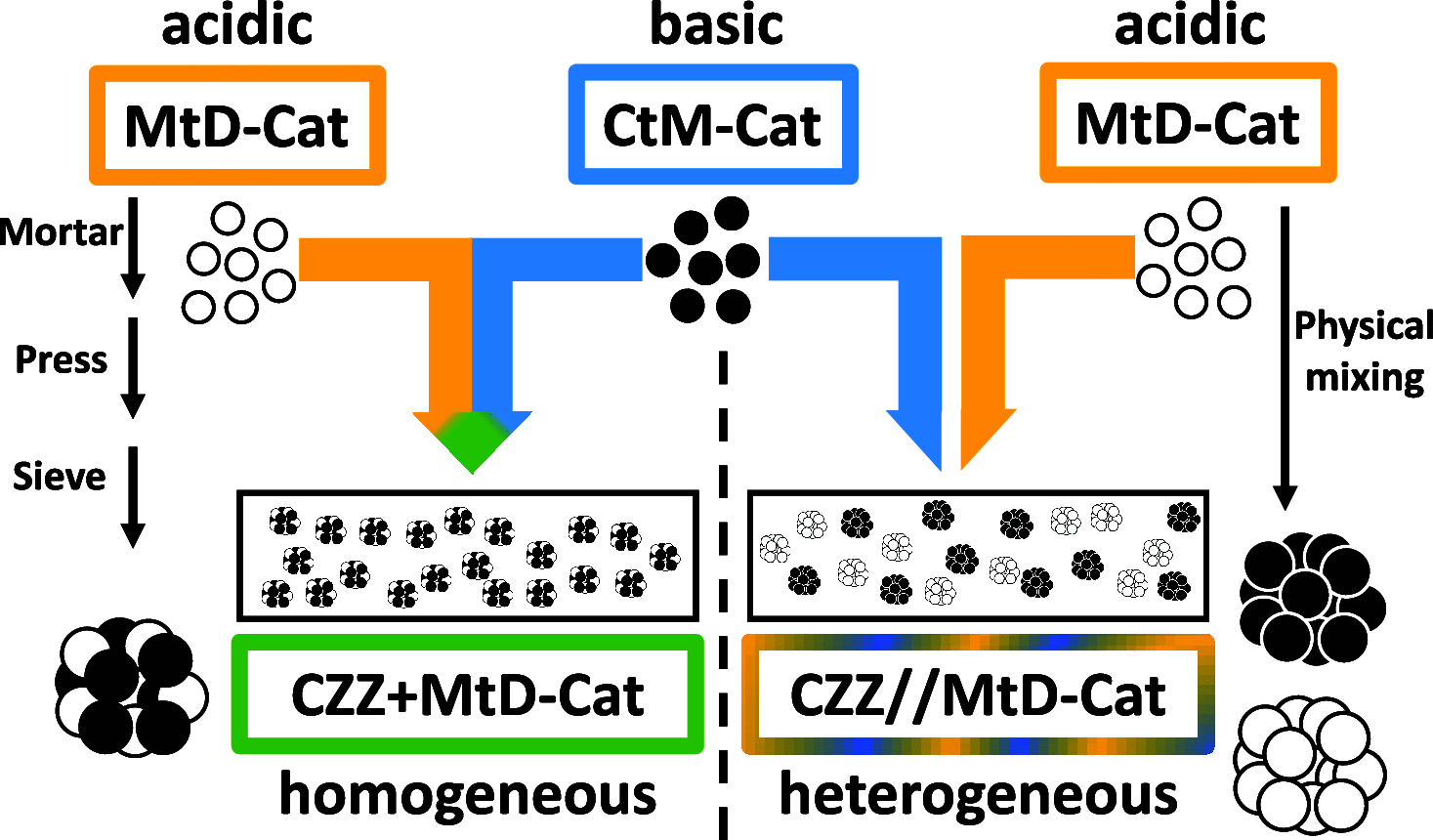
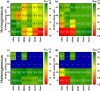
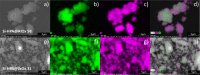
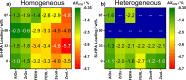
The direct synthesis of dimethyl ether (DME) via CO2 hydrogenation in a single step was studied using an improved class of bifunctional catalysts in a fixed bed reactor (T R: 210-270 °C; 40 bar; gas hourly space velocity (GHSV) 19,800 NL kgcat -1 h-1; ratio CO2/H2/N2 3:9:2). The competitive bifunctional catalysts tested in here consist of a surface-basic copper/zinc oxide/zirconia (CZZ) methanol-producing part and a variable surface-acidic methanol dehydration part and were tested in overall 45 combinations. As dehydration catalysts, zeolites (ferrierite and β-zeolite), alumina, or zirconia were tested alone as well as with a coating of Keggin-type heteropoly acids (HPAs), i.e., silicotungstic or phosphotungstic acid. Two different mixing methods to generate bifunctional catalysts were tested: (i) a single-grain method with intensive intra-particular contact between CZZ and the dehydration catalyst generated by mixing in an agate mortar and (ii) a dual-grain approach relying on physical mixing with low contact. The influence of the catalyst mixing method and HPA loading on catalyst activity and stability was investigated. From these results, a selection of best-performing bifunctional catalysts was investigated in extended measurements (time on stream: 160 h/7 days, T R: 250 and 270 °C; 40 bar; GHSV 19,800 NL kgcat -1 h-1; ratio CO2/H2/N2 3:9:2). Silicotungstic acid-coated bifunctional catalysts showed the highest resilience toward deactivation caused by single-grain preparation and during catalysis. Overall, HPA-coated catalysts showed higher activity and resilience toward deactivation than uncoated counterparts. Dual-grain preparation showed superior performance over single grain. Furthermore, silicotungstic acid coatings with 1 KU nm-2 (Keggin unit per surface area of carrier) on Al2O3 and ZrO2 as carrier materials showed competitive high activity and stability in extended 7-day measurements compared to pure CZZ. Therefore, HPA coating is found to be a well-suited addition to the CO2-to-DME catalyst toolbox.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Schlögl R.Chemical Energy Storage; de Gruyter: Berlin, 2013, 10.1515/9783110266320. - DOI
-
- Styring P.; Dowson G. R. M.; Tozer I. O. Synthetic Fuels Based on Dimethyl Ether as a Future Non-Fossil Fuel for Road Transport From Sustainable Feedstocks. Front. Energy Res. 2021, 9, 66333110.3389/fenrg.2021.663331. - DOI
-
- Wang Y.; Liu H.; Huang Z.; Liu Z. Study on combustion and emission of a dimethyl ether-diesel dual-fuel premixed charge compression ignition combustion engine with LPG (liquefied petroleum gas) as ignition inhibitor. Energy 2016, 96, 278–285. 10.1016/j.energy.2015.12.056. - DOI
-
- Drexler M.; Haltenort P.; Arnold U.; Sauer J. Continuous Synthesis of Oxymethylene Ether Fuels from Dimethyl Ether in a Heterogeneously Catalyzed Liquid Phase Process. Chem. Ing. Tech. 2022, 94, 256–266. 10.1002/cite.202100173. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

