Photocatalytic Degradation of Methylene Blue Using N-Doped ZnO/Carbon Dot (N-ZnO/CD) Nanocomposites Derived from Organic Soybean
- PMID: 37151531
- PMCID: PMC10157678
- DOI: 10.1021/acsomega.2c07546
Photocatalytic Degradation of Methylene Blue Using N-Doped ZnO/Carbon Dot (N-ZnO/CD) Nanocomposites Derived from Organic Soybean
Abstract
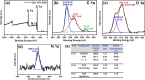
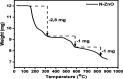
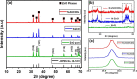
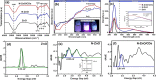
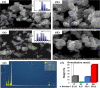
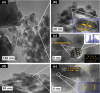
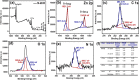
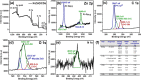
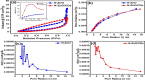
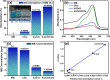
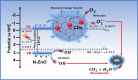
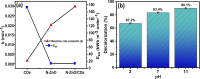
This study reports on successful synthesis of carbon dots (CDs), nitrogen-doped zinc oxide (N-ZnO), and N-ZnO/CD nanocomposites as photocatalysts for degradation of methylene blue. The first part was the synthesis of CDs utilizing a precursor from soybean and ethylenediamine as a dopant by a hydrothermal method. The second part was the synthesis of N-ZnO with urea as the nitrogen dopant carried out by a calcination method in a furnace at 500 °C for 2 h in an N2 atmosphere (5 °C min-1). The third part was the synthesis of N-ZnO/CD nanocomposites. The characteristics of CDs, N-ZnO, and N-ZnO/CD nanocomposites were analyzed through Fourier transform infrared (FTIR), UV-vis absorbance, photoluminescence (PL), high-resolution transmission electron microscopy (HR-TEM), X-ray diffraction (XRD), thermal gravimetry analysis (TGA), field-emission scanning electron microscopy energy-dispersive spectroscopy (FESEM EDS), X-ray photoelectron spectroscopy (XPS), and Brunauer-Emmett-Teller (BET) analysis. Based on the HR-TEM analysis, the CDs had a spherical shape with an average particle size of 4.249 nm. Meanwhile, based on the XRD and HR-TEM characterization, the N-ZnO and N-ZnO/CD nanocomposites have wurtzite hexagonal structures. The materials of N-ZnO and N-ZnO/CD show increased adsorption in the visible light region and low energy gap E g. The E g values of N-ZnO and N-ZnO/CDs were found to be 2.95 and 2.81 eV, respectively, whereas the surface area (S BET) values 3.827 m2 g-1 (N-ZnO) and 3.757 m2 g-1(N-ZnO/CDs) belonged to the microporous structure. In the last part, the photocatalysts of CDs, N-ZnO, and N-ZnO/CD nanocomposites were used for degradation of MB (10 ppm) under UV-B light irradiation pH = 7.04 (neutral) for 60 min at room temperature. The N-ZnO/CD nanocomposites showed a photodegradation efficiency of 83.4% with a kinetic rate of 0.0299 min-1 higher than N-ZnO and CDs. The XRD analysis and FESEM EDS of the N-ZnO/CDs before and after three cycles confirm the stability of the photocatalyst with an MB degradation of 58.2%. These results have clearly shown that the N-ZnO/CD nanocomposites could be used as an ideal photocatalytic material for the decolorization of organic compounds in wastewater.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Bandekar G.; Rajurkar N. S.; Mulla I. S.; Mulik U. P.; Amalnerkar D. P.; Adhyapak P. V. Synthesis, Characterization and Photocatalytic Activity of PVP Stabilized ZnO and Modified ZnO Nanostructures. Appl. Nanosci. 2014, 4, 199.10.1007/s13204-012-0189-2. - DOI
-
- Phillips K. A.; Yau A.; Favela K. A.; Isaacs K. K.; McEachran A.; Grulke C.; Richard A. M.; Williams A. J.; Sobus J. R.; Thomas R. S.; Wambaugh J. F. Suspect Screening Analysis of Chemicals in Consumer Products. Environ. Sci. Technol. 2018, 52, 3125–3135. 10.1021/acs.est.7b04781. - DOI - PMC - PubMed
-
- Senthil Kumar M.; Arunagiri C. Efficient Photocatalytic Degradation of Organic Dyes Using Fe-Doped ZnO Nanoparticles. J. Mater. Sci.: Mater. Electron. 2021, 32, 17925–17935. 10.1007/s10854-021-06328-0. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

