Compatibility and aerosol characteristics of beclomethasone mixed with N-acetylcysteine
- PMID: 37151623
- PMCID: PMC10161602
- DOI: 10.1016/j.heliyon.2023.e15357
Compatibility and aerosol characteristics of beclomethasone mixed with N-acetylcysteine
Abstract
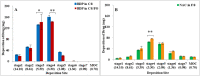
Mixing different kind inhalation medications for simultaneous inhalation is widely used in the treatment of chronic respiratory diseases, and it can minimize the administration time and improve patient adherence. To our knowledge, it is unclear whether beclomethasone (BDP, Clenil®) can bemixed with acetylcysteine (NAC, Fluimucil®), because the in vitro physico-chemical compatibility and aerosol characteristics of the mixture are unknown. In this study, we investigated physical compatibility, including the appearance, pH, osmotic pressure and chemical stability, as well as aerosol characteristics, including particle size corresponding to 10%/50%/90% of the cumulative percentage of total particle volume (X10/X50/X90), volume median droplet diameter (VMD), mass median aerodynamic diameter (MMAD), fine particle fraction (FPF), fine particle dose (FPD) and geometric standard deviation (GSD), delivery rate, and total delivery of the above solutions. After mixing, there were no significant changes in visual appearance, pH, osmolality and drug content of the mixtures at room temperature for 12 h. The FDP of BDP in the mixture decreased by 16.49%, whereas the NAC increased by 10.85%. The delivery rates of BDP and NAC in the mixture decreased by 66.05% and 45.54%, and total delivery increased by 13.20% and 25.29%, respectively. However, the MMAD, FPF, particle size and GSD of the mixture were almost unchanged. We demonstrated that these admixtures are physico-chemically compatible but that coadministration of beclomethasone with acetylcysteine can markedly affect output and aerosol characteristics.
Keywords: Acetylcysteine; Aerosol characteristics; Beclomethasone; Clenil®; Compatibility; Fluimucil®; Stability.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Laube B.L., Dolovich M.B. 66-Aerosols and aerosol drug delivery systems. Middleton's Allergy (Eighth Edition) 2014;1:1066–1082.
-
- Chandel A., et al. Recent advances in aerosolised drug delivery, Biomed. Pharma. 2019;112 - PubMed
-
- Kwok P.C. Advances in inhalation drug delivery. Curr. Pharmaceut. Des. 2021;27:1435. - PubMed
LinkOut - more resources
Full Text Sources

