Increased sinusoidal pressure impairs liver endothelial mechanosensing, uncovering novel biomarkers of portal hypertension
- PMID: 37151732
- PMCID: PMC10154975
- DOI: 10.1016/j.jhepr.2023.100722
Increased sinusoidal pressure impairs liver endothelial mechanosensing, uncovering novel biomarkers of portal hypertension
Abstract
Background & aims: Portal hypertension (PH) is a frequent and severe clinical syndrome associated with chronic liver disease. Considering the mechanobiological effects of hydrostatic pressure and shear stress on endothelial cells, we hypothesised that PH might influence the phenotype of liver sinusoidal endothelial cells (LSECs) during disease progression. The aim of this study was to investigate the effects of increased hydrodynamic pressure on LSECs and to identify endothelial-derived biomarkers of PH.
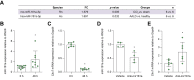
Methods: Primary LSECs were cultured under normal or increased hydrodynamic pressure within a pathophysiological range (1 vs. 12 mmHg) using a microfluidic liver-on-a-chip device. RNA sequencing was used to identify pressure-sensitive genes, which were validated in liver biopsies from two independent cohorts of patients with chronic liver disease with PH (n = 73) and participants without PH (n = 23). Biomarker discovery was performed in two additional independent cohorts of 104 patients with PH and 18 patients without PH.
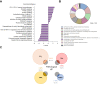
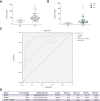
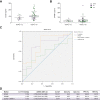
Results: Transcriptomic analysis revealed marked deleterious effect of pathological pressure in LSECs and identified chromobox 7 (CBX7) as a key transcription factor diminished by pressure. Hepatic CBX7 downregulation was validated in patients with PH and significantly correlated with hepatic venous pressure gradient. MicroRNA 181a-5p was identified as pressure-induced upstream regulator of CBX7. Two downstream targets inhibited by CBX7, namely, E-cadherin (ECAD) and serine protease inhibitor Kazal-type 1 (SPINK1), were found increased in the bloodstream of patients with PH and were highly predictive of PH and clinically significant PH.
Conclusions: We characterise the detrimental effects of increased hydrodynamic pressure on the sinusoidal endothelium, identify CBX7 as a pressure-sensitive transcription factor, and propose the combination of two of its reported products as biomarkers of PH.
Impact and implications: Increased pressure in the portal venous system that typically occurs during chronic liver disease (called portal hypertension) is one of the main drivers of related clinical complications, which are linked to a higher risk of death. In this study, we found that pathological pressure has a harmful effect on liver sinusoidal endothelial cells and identified CBX7 as a key protein involved in this process. CBX7 regulates the expression of E-cadherin and SPINK1, and consequently, measuring these proteins in the blood of patients with chronic liver disease allows the prediction of portal hypertension and clinically significant portal hypertension.
Keywords: Endothelial dysfunction; HCV; HVPG; Hepatic haemodynamic; LSEC; Liver cirrhosis; Liver sinusoidal endothelial cells; Mechanobiology; Mechanotransduction; NASH.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing financial interests. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. - PubMed
-
- Jepsen P., Younossi Z.M. The global burden of cirrhosis: a review of disability-adjusted life-years lost and unmet needs. J Hepatol. 2021;75:S3–S13. - PubMed
-
- Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;3383:1749–1761. - PubMed
-
- García-Pagán J.C., Gracia-Sancho J., Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458–461. - PubMed
-
- Gustot T., Stadlbauer V., Laleman W., Alessandria C., Thursz M. Transition to decompensation and acute-on-chronic liver failure: role of predisposing factors and precipitating events. J Hepatol. 2021;75:S36–S48. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

