Determining the impact of professional body recommendations on the screening of acquired carbapenemase-producing Enterobacterales in England
- PMID: 37151818
- PMCID: PMC10160507
- DOI: 10.1016/j.infpip.2023.100281
Determining the impact of professional body recommendations on the screening of acquired carbapenemase-producing Enterobacterales in England
Abstract
Introduction: Acquired carbapenemase-producing Gram-negative bacteria are an increasing public health concern globally and have been mandatory to report in England since October 2020. However, in light of the COVID-19 (SARS-CoV-2) pandemic, the Royal College of Pathologists (RCPath) released new guidance "for reducing the need for screening of CRE (carbapenem-resistant Enterobacterales) […] in low-risk areas", without defining "low risk".
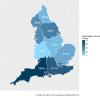
Methods: To assess the impact of the RCPath recommendations on screening of carbapenemase-producing Enterobacterales (CPE), an online Select Survey was sent to all NHS acute hospitals in England. The initial survey distribution was between March and April 2021 and the survey was relaunched between November 2021 and March 2022.
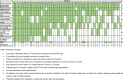
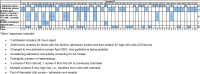
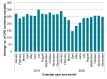
Results: In total, 54 hospitals completed the survey, representing 39.1% of 138 eligible Trusts. All hospitals had a CPE screening policy in place, and the majority of these reflect UKHSA's Framework of actions to contain CPE. Of the 23 hospitals who reported a reduction in CPE screening, only three (13.0%) indicated that this was due to the RCPath recommendations, with 21 (91.3%) indicating that there had been a natural reduction in the number of patients admitted to the Trust who would have previously been screened due to the COVID-19 pandemic.
Conclusion: For most surveyed hospitals, CPE screening was not reduced due to the RCPath recommendations. However, the results highlighted that there is a large amount of individual variation in CPE screening practices and diagnostic testing between hospitals.
Keywords: COVID-19 pandemic; CPE; Carbapenemase-producing; Enterobacterales; Policy; Screening.
Crown Copyright © 2023 Published by Elsevier Ltd on behalf of The Healthcare Infection Society.
Figures
References
-
- Department of Health and Social Care . 2019. Tackling antimicrobial resistance 2019 to 2024: the UK’s 5-year national action plan; pp. 27–30. January.
-
- UK Health Security Agency . 2020. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2020-2021.
-
- Doshi N. 2022. Bacteriology reference department user manual.
-
- Department of Health and Social Care . 2020. The Health protection (notification) (amendment) (No.2) Regulations 2020.
-
- Royal College of Pathologists . NHS England and NHS Improvement; UK: 2020. Prioritisation/deferral of pathology laboratory work (in light of SARS-CoV-2 (COVID19) epidemic.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

