Cytochalasin D restores nuclear size acting on F-actin and IZUMO1 localization in low-quality spermatozoa
- PMID: 37151878
- PMCID: PMC10158014
- DOI: 10.7150/ijbs.77166
Cytochalasin D restores nuclear size acting on F-actin and IZUMO1 localization in low-quality spermatozoa
Abstract
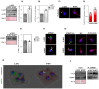
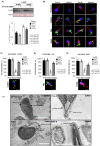
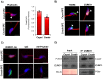
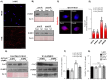
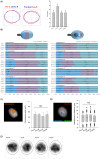
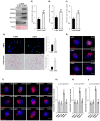
In spermatozoa, the nuclear F-actin supports the acroplaxome, a subacrosomal structure involved in the correct exposure of several acrosomal membrane proteins; among them, the glycoprotein IZUMO1 is the major protein involved in sperm-oocyte fusion. Nuclear F-actin is also involved in sperm head shaping and chromosome compartmentalization. To date, few notions regarding the bivalent role of F-actin on sperm chromatin organization and IZUMO1 positioning have been reported. In our work, we characterized subcellular organization of F-actin in human high- and low-quality spermatozoa (A- and B-SPZ), respectively, showing that F-actin over-expression in sperm head of B-SPZ affected IZUMO1 localization. A correct IZUMO1 repositioning following in vitro induction of F-actin depolymerization, by cytochalasin D treatment, occurred. Interestingly, F-actin depolymerization was also associated with a correct acrosome repositioning, thus to favor a proper acrosome reaction onset, with changes in sperm nuclear size parameters and histone acetylation rate reaching high-quality conditions. In conclusion, the current work shows a key role of F-actin in the control of IZUMO1 localization as well as chromatin remodeling and acetylation events.
Keywords: F-actin; IZUMO1; chromosomes territories; histone acetylation; sperm quality.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, Breitbart H. Remodeling of the Actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol Reprod. 2003;68:837–845. doi:10.1095/biolreprod.102.009233. - PubMed
-
- Howes ES, Hurst SM, Jones R. Actin and Actin binding proteins in bovine spermatozoa: Potential role in membrane remodeling and intracellular signaling during epididymal maturation and the acrosome reaction. J Androl. 2001;22:62–72. doi.org/10.1002/j.1939-4640.2001.tb02154.x. - PubMed
-
- Breitbart H, Finkelstein M. Actin cytoskeleton and sperm function. Biochem Biophys Res Commun. 2018;506:372–377. doi: 10.1016/j.bbrc.2017.11.001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

