Agomelatine in the treatment of anhedonia, somatic symptoms, and sexual dysfunction in major depressive disorder
- PMID: 37151978
- PMCID: PMC10157485
- DOI: 10.3389/fpsyt.2023.1115008
Agomelatine in the treatment of anhedonia, somatic symptoms, and sexual dysfunction in major depressive disorder
Abstract
Objective: This study evaluated the treatment outcomes of agomelatine on anhedonic state, anxiety/somatic symptoms, and sexual function in Chinese patients with major depressive disorder (MDD).
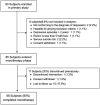
Method: In total, 93 adult patients with MDD were enrolled, and 68 of them were included in a prospective, open-label, multicenter clinical study. All patients received agomelatine monotherapy during a 9-week treatment phase. The effectiveness of the treatment was reflected by the improvement of anhedonia and somatic symptoms based on the 17-item Hamilton Depression Rating Scale (HAMD-17). In addition, the Arizona Sexual Dysfunction Scale (ASEX), Sheehan Disability Scale (SDS), and Short Form of Quality-of-Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q-SF) were administered to all participants at baseline and at the 3-, 6-, and 9-week follow-ups.
Results: After 9 weeks of treatment with agomelatine, the response and remission rates were 73.5% and 39.7%, respectively. Somatic symptoms significantly improved at week 9 (p < 0.001), and significant effects were also observed on the HAMD anhedonia items (p < 0.001). The patients exhibited lower levels of disease severity (the SDS score dropped from 15.52 ± 4.7 to 7.09 ± 5.62 at week 9; the ASEX score dropped from 21.89 ± 4.06 to 16.19 ± 4.79, p < 0.001) and higher levels of QOL (the Q-LES-Q-SF score dropped from 41.02 ± 5.99 to 50.49 ± 8.57, p < 0.001) during the follow-up. Furthermore, treatment with agomelatine improved depressive symptoms without causing serious adverse events.
Conclusion: These analyses indicate that agomelatine is a treatment option for improving anhedonic status, anxiety/somatic symptoms, and sexual dysfunction in MDD patients.
Keywords: agomelatine; anhedonia; major depressive disorder; sexual dysfunction; somatic symptoms.
Copyright © 2023 Huang, Xie, Lyu, Fu, Zhao, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization. (2021). Depression. Geneva: World Health Organization. Available online at: https://www.who.int/health-topics/depression#tab=tab/1 (accessed Jan 13, 2021).
LinkOut - more resources
Full Text Sources

