Targeted locus amplification to develop robust patient-specific assays for liquid biopsies in pediatric solid tumors
- PMID: 37152023
- PMCID: PMC10157037
- DOI: 10.3389/fonc.2023.1124737
Targeted locus amplification to develop robust patient-specific assays for liquid biopsies in pediatric solid tumors
Abstract
Background: Liquid biopsies combine minimally invasive sample collection with sensitive detection of residual disease. Pediatric malignancies harbor tumor-driving copy number alterations or fusion genes, rather than recurrent point mutations. These regions contain tumor-specific DNA breakpoint sequences. We investigated the feasibility to use these breakpoints to design patient-specific markers to detect tumor-derived cell-free DNA (cfDNA) in plasma from patients with pediatric solid tumors.
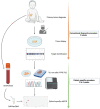
Materials and methods: Regions of interest (ROI) were identified through standard clinical diagnostic pipelines, using SNP array for CNAs, and FISH or RT-qPCR for fusion genes. Using targeted locus amplification (TLA) on tumor organoids grown from tumor material or targeted locus capture (TLC) on FFPE material, ROI-specific primers and probes were designed, which were used to design droplet digital PCR (ddPCR) assays. cfDNA from patient plasma at diagnosis and during therapy was analyzed.
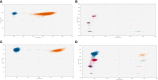
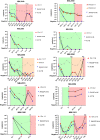
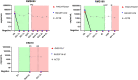
Results: TLA was performed on material from 2 rhabdomyosarcoma, 1 Ewing sarcoma and 3 neuroblastoma. FFPE-TLC was performed on 8 neuroblastoma tumors. For all patients, at least one patient-specific ddPCR was successfully designed and in all diagnostic plasma samples the patient-specific markers were detected. In the rhabdomyosarcoma and Ewing sarcoma patients, all samples after start of therapy were negative. In neuroblastoma patients, presence of patient-specific markers in cfDNA tracked tumor burden, decreasing during induction therapy, disappearing at complete remission and re-appearing at relapse.
Conclusion: We demonstrate the feasibility to determine tumor-specific breakpoints using TLA/TLC in different pediatric solid tumors and use these for analysis of cfDNA from plasma. Considering the high prevalence of CNAs and fusion genes in pediatric solid tumors, this approach holds great promise and deserves further study in a larger cohort with standardized plasma sampling protocols.
Keywords: TLA; cell-free DNA (cfDNA); liquid biopsy; neuroblastoma; paediatric cancer; patient-specific ddPCR.
Copyright © 2023 van Zogchel, Lak, Gelineau, Sergeeva, Stelloo, Swennenhuis, Feitsma, van Min, Splinter, Bleijs, Groot Koerkamp, Breunis, Meister, Kholossy, Holstege, Molenaar, de Leng, Stutterheim, van der Schoot and Tytgat.
Conflict of interest statement
IS, ESt, JSw, HF, MM, ESp are employees of Cergentis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures

Similar articles
-
The feasibility of using liquid biopsies as a complementary assay for copy number aberration profiling in routinely collected paediatric cancer patient samples.Eur J Cancer. 2022 Jan;160:12-23. doi: 10.1016/j.ejca.2021.09.022. Epub 2021 Nov 16. Eur J Cancer. 2022. PMID: 34794856
-
Case series on clinical applications of liquid biopsy in pediatric solid tumors: towards improved diagnostics and disease monitoring.Front Oncol. 2023 Aug 17;13:1209150. doi: 10.3389/fonc.2023.1209150. eCollection 2023. Front Oncol. 2023. PMID: 37664065 Free PMC article.
-
Somatic Copy Number Alteration in Circulating Tumor DNA for Monitoring of Pediatric Patients with Cancer.Biomedicines. 2023 Apr 3;11(4):1082. doi: 10.3390/biomedicines11041082. Biomedicines. 2023. PMID: 37189699 Free PMC article.
-
The pitfalls and promise of liquid biopsies for diagnosing and treating solid tumors in children: a review.Eur J Pediatr. 2020 Feb;179(2):191-202. doi: 10.1007/s00431-019-03545-y. Epub 2020 Jan 3. Eur J Pediatr. 2020. PMID: 31897843 Free PMC article. Review.
-
Using circulating cell-free DNA to monitor personalized cancer therapy.Crit Rev Clin Lab Sci. 2017 May;54(3):205-218. doi: 10.1080/10408363.2017.1299683. Epub 2017 Apr 10. Crit Rev Clin Lab Sci. 2017. PMID: 28393575 Review.
Cited by
-
Serially Quantifying TERT Rearrangement Breakpoints in ctDNA Enables Minimal Residual Disease Monitoring in Patients with Neuroblastoma.Cancer Res Commun. 2025 Jan 1;5(1):167-177. doi: 10.1158/2767-9764.CRC-24-0569. Cancer Res Commun. 2025. PMID: 39760332 Free PMC article.
-
A comprehensive overview of liquid biopsy applications in pediatric solid tumors.NPJ Precis Oncol. 2024 Aug 3;8(1):172. doi: 10.1038/s41698-024-00657-z. NPJ Precis Oncol. 2024. PMID: 39097671 Free PMC article. Review.
References
-
- Chisholm JC, Marandet J, Rey A, Scopinaro M, de Toledo JS, Merks JH, et al. . Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: a nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol (2011) 29(10):1319–25. doi: 10.1200/JCO.2010.32.1984 - DOI - PubMed
LinkOut - more resources
Full Text Sources

