Kruppel-like factor 8 regulates triple negative breast cancer stem cell-like activity
- PMID: 37152043
- PMCID: PMC10155275
- DOI: 10.3389/fonc.2023.1141834
Kruppel-like factor 8 regulates triple negative breast cancer stem cell-like activity
Abstract
Introduction: Breast tumor development is regulated by a sub-population of breast cancer cells, termed cancer stem-like cells (CSC), which are capable of self-renewing and differentiating, and are involved in promoting breast cancer invasion, metastasis, drug resistance and relapse. CSCs are highly adaptable, capable of reprogramming their own metabolism and signaling activity in response to stimuli within the tumor microenvironment. Recently, the nutrient sensor O-GlcNAc transferase (OGT) and O-GlcNAcylation was shown to be enriched in CSC populations, where it promotes the stemness and tumorigenesis of breast cancer cells in vitro and in vivo. This enrichment was associated with upregulation of the transcription factor Kruppel-like-factor 8 (KLF8) suggesting a potential role of KLF8 in regulating CSCs properties.
Methods: Triple-negative breast cancer cells were genetically modified to generate KLF8 overexpressing or KLF8 knock-down cells. Cancer cells, control or with altered KLF8 expression were analyzed to assess mammosphere formation efficiency, CSCs frequency and expression of CSCs factors. Tumor growth in vivo of control or KLF8 knock-down cells was assessed by fat-pad injection of these cell in immunocompromised mice.
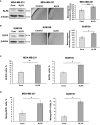
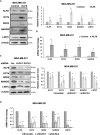
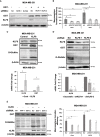
Results: Here, we show that KLF8 is required and sufficient for regulating CSC phenotypes and regulating transcription factors SOX2, NANOG, OCT4 and c-MYC. KLF8 levels are associated with chemoresistance in triple negative breast cancer patients and overexpression in breast cancer cells increased paclitaxel resistance. KLF8 and OGT co-regulate each other to form a feed-forward loop to promote CSCs phenotype and mammosphere formation of breast cancer cells.
Discussion: These results suggest a critical role of KLF8 and OGT in promoting CSCs and cancer progression, that may serve as potential targets for developing strategy to target CSCs specifically.
Keywords: KLF8; O-GlcNAc; OGT; breast cancer; cancer stem cell; self-renewal.
Copyright © 2023 Le Minh, Esquea, Dhameliya, Merzy, Lee, Ball and Reginato.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
O-GlcNAc Transferase Regulates Cancer Stem-like Potential of Breast Cancer Cells.Mol Cancer Res. 2020 Apr;18(4):585-598. doi: 10.1158/1541-7786.MCR-19-0732. Epub 2020 Jan 23. Mol Cancer Res. 2020. PMID: 31974291 Free PMC article.
-
GATAD2B O-GlcNAcylation Regulates Breast Cancer Stem-like Potential and Drug Resistance.Cells. 2025 Mar 8;14(6):398. doi: 10.3390/cells14060398. Cells. 2025. PMID: 40136647 Free PMC article.
-
Hyperglycemia and O-GlcNAc transferase activity drive a cancer stem cell pathway in triple-negative breast cancer.Cancer Cell Int. 2023 May 25;23(1):102. doi: 10.1186/s12935-023-02942-6. Cancer Cell Int. 2023. PMID: 37231419 Free PMC article.
-
Reporters of Cancer Stem Cells as a Tool for Drug Discovery.Front Oncol. 2021 Apr 22;11:669250. doi: 10.3389/fonc.2021.669250. eCollection 2021. Front Oncol. 2021. PMID: 33968778 Free PMC article. Review.
-
The Role of Steroid Hormones in Breast and Effects on Cancer Stem Cells.Curr Stem Cell Rep. 2018;4(1):81-94. doi: 10.1007/s40778-018-0114-z. Epub 2018 Mar 13. Curr Stem Cell Rep. 2018. PMID: 29600163 Free PMC article. Review.
Cited by
-
Possible Strategies to Reduce the Tumorigenic Risk of Reprogrammed Normal and Cancer Cells.Int J Mol Sci. 2024 May 9;25(10):5177. doi: 10.3390/ijms25105177. Int J Mol Sci. 2024. PMID: 38791215 Free PMC article. Review.
-
Prognostic and clinicopathological roles of circular RNA expression in chemoresistance in head and neck squamous cell carcinoma: a systematic review.Front Pharmacol. 2025 Mar 19;16:1502107. doi: 10.3389/fphar.2025.1502107. eCollection 2025. Front Pharmacol. 2025. PMID: 40176914 Free PMC article.
-
Mitochondria in Cancer Stem Cells: From an Innocent Bystander to a Central Player in Therapy Resistance.Stem Cells Cloning. 2023 Aug 23;16:19-41. doi: 10.2147/SCCAA.S417842. eCollection 2023. Stem Cells Cloning. 2023. PMID: 37641714 Free PMC article. Review.
-
The roles of OGT and its mechanisms in cancer.Cell Biosci. 2024 Sep 16;14(1):121. doi: 10.1186/s13578-024-01301-w. Cell Biosci. 2024. PMID: 39285476 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

