Trans-cinnamaldehyde-related overproduction of benzoic acid and oxidative stress on Arabidopsis thaliana
- PMID: 37152151
- PMCID: PMC10160683
- DOI: 10.3389/fpls.2023.1157309
Trans-cinnamaldehyde-related overproduction of benzoic acid and oxidative stress on Arabidopsis thaliana
Abstract
Introduction: Trans-cinnamaldehyde is a specialised metabolite that naturally occurs in plants of the Lauraceae family. This study focused on the phytotoxic effects of this compound on the morphology and metabolism of Arabidopsis thaliana seedlings.
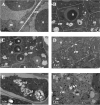
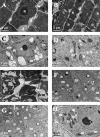
Material and methods: To evaluate the phytotoxicity of trans-cinnamaldehyde, a dose-response curve was first performed for the root growth process in order to calculate the reference inhibitory concentrations IC50 and IC80 (trans-cinnamaldehyde concentrations inducing a 50% and 80% inhibition, respectively). Subsequently, the structure and ultrastructure of the roots treated with the compound were analysed by light and electron microscopy. Based on these results, the following assays were carried out to in depth study the possible mode of action of the compound: antiauxinic PCIB reversion bioassay, determination of mitochondrial membrane potential, ROS detection, lipid peroxidation content, hormone quantification, in silico studies and gene expression of ALDH enzymes.
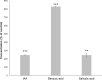
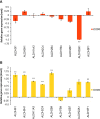
Results: Trans-cinnamaldehyde IC50 and IC80 values were as low as 46 and 87 μM, reducing the root growth and inducing the occurrence of adventitious roots. At the ultrastructural level, the compound caused alterations to the mitochondria, which were confirmed by detection of the mitochondrial membrane potential. The morphology observed after the treatment (i.e., appearance of adventitious roots) suggested a possible hormonal mismatch at the auxin level, which was confirmed after PCIB bioassay and hormone quantification by GC-MS. The addition of the compound caused an increase in benzoic, salicylic and indoleacetic acid content, which was related to the increased gene expression of the aldehyde dehydrogenase enzymes that can drive the conversion of trans-cinnamaldehyde to cinnamic acid. Also, an increase of ROS was also observed in treated roots. The enzyme-compound interaction was shown to be stable over time by docking and molecular dynamics assays.
Discussion: The aldehyde dehydrogenases could drive the conversion of trans-cinnamaldehyde to cinnamic acid, increasing the levels of benzoic, salicylic and indoleacetic acids and causing the oxidative stress symptoms observed in the treated seedlings. This would result into growth and development inhibition of the trans-cinnamaldehyde-treated seedlings and ultimately in their programmed-cell-death.
Keywords: ALDHs; Arabidopsis; cinnamic acid; hormones; oxidative stress; trans-cinnamaldehyde.
Copyright © 2023 López-González, Ferradás, Araniti, Graña, Hermida-Ramón, González, Teijeira, Rey, Reigosa and Sánchez-Moreiras.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Ahmad F., Singh A., Kamal A. (2019). “Salicylic acid-mediated defense mechanisms to abiotic stress tolerance”, in Plant signaling molecules: role and regulation under stressful environments. Eds. Khan M. I. R., Reddy P. S., Ferrante A., Khan N. A. (Duxford, United Kingdom:Woodhead Publishing; ), 355–369. doi: 10.1016/B978-0-12-816451-8.00022-8 - DOI
-
- Araniti F., Lupini A., Mauceri A., Zumbo A., Sunseri F., Abenavoli M. R. (2018. b). The allelochemical trans-cinnamic acid stimulates salicylic acid production and galactose pathway in maize leaves: a potential mechanism of stress tolerance. Plant Physiol. Biochem. 128, 32–40. doi: 10.1016/j.plaphy.2018.05.006 - DOI - PubMed
-
- Bailey K. L. (2014). “The bioherbicide approach to weed control using plant pathogens”, in Integrated pest management: current concepts and ecological perspective. Ed. Abrol D. (San Diego, USA.:Academic Press; ), 245–266. doi: 10.1016/B978-0-12-398529-3.00014-2 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

