Streamlining the interface between electronics and neural systems for bidirectional electrochemical communication
- PMID: 37152246
- PMCID: PMC10155913
- DOI: 10.1039/d3sc00338h
Streamlining the interface between electronics and neural systems for bidirectional electrochemical communication
Abstract
Seamless neural interfaces conjoining neurons and electrochemical devices hold great potential for highly efficient signal transmission across neural systems and the external world. Signal transmission through chemical sensing and stimulation via electrochemistry is remarkable because communication occurs through the same chemical language of neurons. Emerging strategies based on synaptic interfaces, iontronics-based neuromodulation, and improvements in selective neurosensing techniques have been explored to achieve seamless integration and efficient neuro-electronics communication. Synaptic interfaces can directly exchange signals to and from neurons, in a similar manner to that of chemical synapses. Hydrogel-based iontronic chemical delivery devices are operationally compatible with neural systems for improved neuromodulation. In this perspective, we explore developments to improve the interface between neurons and electrodes by targeting neurons or sub-neuronal regions including synapses. Furthermore, recent progress in electrochemical neurosensing and iontronics-based chemical delivery is examined.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
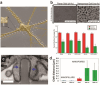

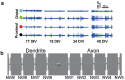
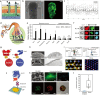
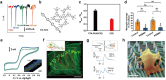





References
-
- Palade G. E. Palay S. L. Anat. Rec. 1954;118:335.
Publication types
LinkOut - more resources
Full Text Sources

