Light-induced homolysis of copper(ii)-complexes - a perspective for photocatalysis
- PMID: 37152247
- PMCID: PMC10155906
- DOI: 10.1039/d3sc00388d
Light-induced homolysis of copper(ii)-complexes - a perspective for photocatalysis
Abstract
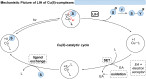
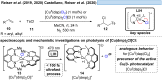
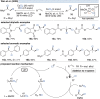
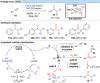
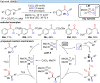
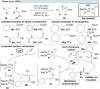
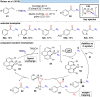
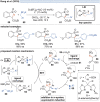
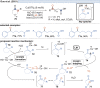
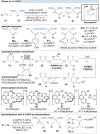
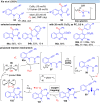
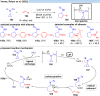
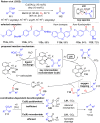
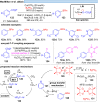
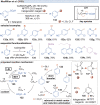
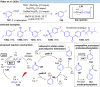
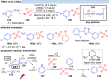
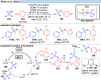
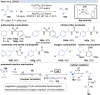
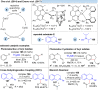
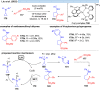
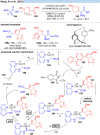
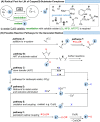
Over the past decade, photocatalysis has developed into a powerful strategy for the selective functionalization of molecules through radical intermediates. Besides the well-established iridium- or ruthenium-based photocatalysts, which ideally fulfill the requirements for a photocatalyst, such as long excited-state lifetimes and photostability, the shift towards earth-abundant metal-based photocatalysts has so far been less explored. The concept of light-induced homolysis (LIH) for generating radicals has recently gained significant interest as a new platform for inducing photoreactions with earth-abundant 3d-metal complexes despite only having excited-state lifetimes in the low nanosecond range or even below. Cu(ii)-complexes play a prominent role in exploiting this concept, which will be discussed by showcasing recent developments in organic synthesis with a view to identifying the future prospects of this growing field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Shaw M. H. Twilton J. MacMillan D. W. C. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. - DOI - PMC - PubMed
- Marzo L. Pagire S. K. Reiser O. König B. Angew. Chem., Int. Ed. 2018;57:10034–10072. doi: 10.1002/anie.201709766. - DOI - PubMed
- Bell J. D. Murphy J. A. Chem. Soc. Rev. 2021;50:9540–9685. doi: 10.1039/D1CS00311A. - DOI - PubMed
- Melchiorre P. Chem. Rev. 2022;122:1483–1484. doi: 10.1021/acs.chemrev.1c00993. - DOI - PubMed
-
- Ramaiah M. Tetrahedron. 1987;43:3541–3676. doi: 10.1016/S0040-4020(01)86853-8. - DOI
-
- Curran D. P., Porter N. A. and Giese B., Stereochemistry of Radical Reactions. Concepts, Guidelines, and Synthetic Applications with a Foreword by Ernest L. Eliel, Wiley-VCH, Weinheim, 1st edn, 2008
- Jasperse C. P. Curran D. P. Fevig T. L. Chem. Rev. 1991;91:1237–1286. doi: 10.1021/cr00006a006. - DOI
- Studer A. Curran D. P. Angew. Chem., Int. Ed. 2016;55:58–102. doi: 10.1002/anie.201505090. - DOI - PubMed
-
- Haxel G. B., Hedrick J. B. and Orris G. J., Rare Earth Elements-Critical Resources for High Technology, United States Geological Survey, Reston, VA, USA, 2002
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

