Exhaled aerosols among PCR-confirmed SARS-CoV-2-infected children
- PMID: 37152322
- PMCID: PMC10160682
- DOI: 10.3389/fped.2023.1156366
Exhaled aerosols among PCR-confirmed SARS-CoV-2-infected children
Abstract
Background: Available data on aerosol emissions among children and adolescents during spontaneous breathing are limited. Our aim was to gain insight into the role of children in the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and whether aerosol measurements among children can be used to help detect so-called superspreaders-infected individuals with extremely high numbers of exhaled aerosol particles.
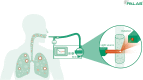
Methods: In this prospective study, the aerosol concentrations of SARS-CoV-2 PCR-positive and SARS-CoV-2 PCR-negative children and adolescents (2-17 years) were investigated. All subjects were asked about their current health status and medical history. The exhaled aerosol particle counts of PCR-negative and PCR-positive subjects were measured using the Resp-Aer-Meter (Palas GmbH, Karlsruhe, Germany) and compared using linear regression.
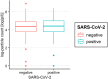
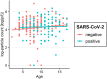
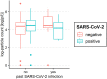
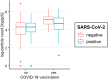
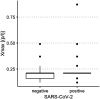
Results: A total of 250 children and adolescents were included in this study, 105 of whom were SARS-CoV-2 positive and 145 of whom were SARS-CoV-2 negative. The median age in both groups was 9 years (IQR 7-11 years). A total of 124 (49.6%) participants were female, and 126 (50.4%) participants were male. A total of 81.9% of the SARS-CoV-2-positive group had symptoms of viral infection. The median particle count of all individuals was 79.55 particles/liter (IQR 44.55-141.15). There was a tendency for older children to exhale more particles (1-5 years: 79.54 p/L; 6-11 years: 77.96 p/L; 12-17 years: 98.63 p/L). SARS-CoV-2 PCR status was not a bivariate predictor (t = 0.82, p = 0.415) of exhaled aerosol particle count; however, SARS-CoV-2 status was shown to be a significant predictor in a multiple regression model together with age, body mass index (BMI), COVID-19 vaccination, and past SARS-CoV-2 infection (t = 2.81, p = 0.005). COVID-19 vaccination status was a highly significant predictor of exhaled aerosol particles (p < .001).
Conclusion: During SARS-CoV-2 infection, children and adolescents did not have elevated aerosol levels. In addition, no superspreaders were found.
Keywords: COVID-19 vaccination; SARS-CoV-2 infection; aerosols; children; omicron.
© 2023 Schuchmann, Scheuch, Naumann, Keute, Lücke, Zielen and Brinkmann.
Conflict of interest statement
SZ reports, received grants from Palas GmbH, grants and personal fees from Allergy Therapeutics GmbH, grants and personal fees from Böhringer Ingelheim, personal fees from Novartis GmbH, personal fees from Lofarma GmbH, personal fees from IMS HEALTH GmbH & Co. OHG, personal fees from GSK, personal fees from Stallergen, personal fees from Engelhard Arzeneimittel, personal fees from Sanofi-Pasteur, personal fees from AstraZeneca, personal fees from Erydel, outside the submitted work. GS was employed by GS Bio-Inhalation GmbH, Headquarters & Logistics. RN was employed by AspiAir GmbH. MK was employed by Independent Statistical Consultant. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Who coronavirus (Covid-19) dashboard | who coronavirus (Covid-19) dashboard with vaccination data (2022). Available at: https://Covid19.Who.Int/?Adgroupsurvey={Adgroupsurvey}&Gclid=Cjwkcaia7vw... (Accessed December 17, 2022).
LinkOut - more resources
Full Text Sources
Miscellaneous

