Organic and inorganic nanomaterials: fabrication, properties and applications
- PMID: 37152571
- PMCID: PMC10162010
- DOI: 10.1039/d3ra01421e
Organic and inorganic nanomaterials: fabrication, properties and applications
Abstract
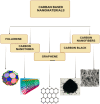
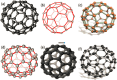
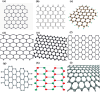
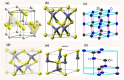
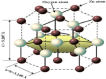
Nanomaterials and nanoparticles are a burgeoning field of research and a rapidly expanding technology sector in a wide variety of application domains. Nanomaterials have made exponential progress due to their numerous uses in a variety of fields, particularly the advancement of engineering technology. Nanoparticles are divided into various groups based on the size, shape, and structural morphology of their bodies. The 21st century's defining feature of nanoparticles is their application in the design and production of semiconductor devices made of metals, metal oxides, carbon allotropes, and chalcogenides. For the researchers, these materials then opened a new door to a variety of applications, including energy storage, catalysis, and biosensors, as well as devices for conversion and medicinal uses. For chemical and thermal applications, ZnO is one of the most stable n-type semiconducting materials available. It is utilised in a wide range of products, from luminous materials to batteries, supercapacitors, solar cells to biomedical photocatalysis sensors, and it may be found in a number of forms, including pellets, nanoparticles, bulk crystals, and thin films. The distinctive physiochemical characteristics of semiconducting metal oxides are particularly responsible for this. ZnO nanostructures differ depending on the synthesis conditions, growth method, growth process, and substrate type. A number of distinct growth strategies for ZnO nanostructures, including chemical, physical, and biological methods, have been recorded. These nanostructures may be synthesized very simply at very low temperatures. This review focuses on and summarizes recent achievements in fabricating semiconductor devices based on nanostructured materials as 2D materials as well as rapidly developing hybrid structures. Apart from this, challenges and promising prospects in this research field are also discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures





























References
-
- Khan R. Shigidi I. Otaibi S. A. Althubeiti K. Abdullaev S. S. Rahman N. sohail M. Khan A. Iqbal S. Del Rosso T. Zaman Q. Khan A. Room temperature dilute magnetic semiconductor response in (Gd, Co) co-doped ZnO for efficient spintronics applications. RSC Adv. 2022;12:36126–36137. doi: 10.1039/D2RA06637H. - DOI - PMC - PubMed
-
- United Nations, Questions About Nanotechnology, 2012, https://www.epa.gov/chemical-research/research-nanomaterials, accessed Aug 21, 2014
-
- Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology, Federal Drug Administration, USA, 2011, https://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.html, accessed Jan 25, 2016
-
- ISO/TS 80004-1:2010, Nanotechnology – Vocabulary – Part 1: Core Terms, International Organization for Standardization, Geneva, Switzerland, 2010, https://www.iso.org/standard/51240.html, accessed July 17, 2017
-
- Bleeker E. A. J., Cassee F. R., Geertsma R. E., de Jong W. H., Heugens E. H. W., Koers-Jacquemijns M., van De Meent D., Oomen A. G., Popma J., Rietveld A. G. and Wijnhoven S. W. P., Interpretation and implications of the European Commission's definition on nanomaterials; Letter report 601358001, RIVM, Bilthoven, Netherlands, 2012
Publication types
LinkOut - more resources
Full Text Sources

