Chitosan can improve antimicrobial treatment independently of bacterial lifestyle, biofilm biomass intensity and antibiotic resistance pattern in non-aureus staphylococci (NAS) isolated from bovine clinical mastitis
- PMID: 37152721
- PMCID: PMC10162019
- DOI: 10.3389/fmicb.2023.1167693
Chitosan can improve antimicrobial treatment independently of bacterial lifestyle, biofilm biomass intensity and antibiotic resistance pattern in non-aureus staphylococci (NAS) isolated from bovine clinical mastitis
Abstract
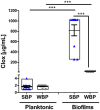
Bovine mastitis is the most frequent and costly disease that affects dairy cattle. Non-aureus staphylococci (NAS) are currently one of the main pathogens associated with difficult-to-treat intramammary infections. Biofilm is an important virulence factor that can protect bacteria against antimicrobial treatment and prevent their recognition by the host's immune system. Previously, we found that chronic mastitis isolates which were refractory to antibiotic therapy developed strong biofilm biomass. Now, we evaluated the influence of biofilm biomass intensity on the antibiotic resistance pattern in strong and weak biofilm-forming NAS isolates from clinical mastitis. We also assessed the effect of cloxacillin (Clx) and chitosan (Ch), either alone or in combination, on NAS isolates with different lifestyles and abilities to form biofilm. The antibiotic resistance pattern was not the same in strong and weak biofilm producers, and there was a significant association (p ≤ 0.01) between biofilm biomass intensity and antibiotic resistance. Bacterial viability assays showed that a similar antibiotic concentration was effective at killing both groups when they grew planktonically. In contrast, within biofilm the concentrations needed to eliminate strong producers were 16 to 128 times those needed for weak producers, and more than 1,000 times those required for planktonic cultures. Moreover, Ch alone or combined with Clx had significant antimicrobial activity, and represented an improvement over the activity of the antibiotic on its own, independently of the bacterial lifestyle, the biofilm biomass intensity or the antibiotic resistance pattern. In conclusion, the degree of protection conferred by biofilm against antibiotics appears to be associated with the intensity of its biomass, but treatment with Ch might be able to help counteract it. These findings suggest that bacterial biomass should be considered when designing new antimicrobial therapies aimed at reducing antibiotic concentrations while improving cure rates.
Keywords: antibiotic resistance; biofilm; biofilm and antibiotic resistance association; chitosan; cloxacillin; non-aureus staphylococcus.
Copyright © 2023 Breser, Tisera, Orellano, Bohl, Isaac, Bianco and Porporatto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



References
-
- Aidara-Kane A., Angulo F. J., Conly J. M., Minato Y., Silbergeld E. K., McEwen S. A., Collignon P. J., WHO Guideline Development Group . (2018). World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control. 7:7. doi: 10.1186/s13756-017-0294-9 - DOI - PMC - PubMed
-
- Asli A., Brouillette E., Ster C., Ghinet M. G., Brzezinski R., Lacasse P., et al. . (2017). Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS One 12:e0176988. doi: 10.1371/journal.pone.0176988, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

