NIVOLUMAB ASSOCIATED ENDOCRINE ABNORMALITIES: CHALLENGING CASES FROM A REFERENCE CLINIC
- PMID: 37152877
- PMCID: PMC10162826
- DOI: 10.4183/aeb.2022.516
NIVOLUMAB ASSOCIATED ENDOCRINE ABNORMALITIES: CHALLENGING CASES FROM A REFERENCE CLINIC
Abstract
Background: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced cancers. Antibodies directed against programmed cell death receptor 1 (PD-1) interrupt the ability of the cancerous cell to depress the immune system.
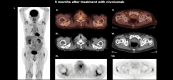
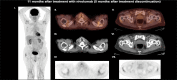
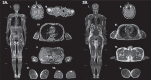
Methods and results: We report three patients who developed different endocrine abnormalities after treatment with nivolumab, a monoclonal antibody directed against PD-1. First, we report a 76-year-old male presenting with generalized fat loss after treatment with nivolumab which predominantly affected his face and trunk. Second, we described the development of thyroiditis that presented with thyrotoxicosis and the expression of thyroid-stimulating hormone receptor antibodies (TRAb). Finally, we observed the emergence of adrenal insufficiency due to hypophysitis in another case.
Conclusion: Although immune checkpoint inhibitors are an effective anticancer treatment modality, adverse effects are evident that can affect the endocrine system. These adverse events may relate to different endocrine systems that include the thyroid and pituitary glands. Also, acquired generalized lipodystrophy should be suspected in patients developing unusual fat loss after treatment with ICIs.
Keywords: Grave’s disease; adrenal insufficiency; hypophysitis; lipodystrophy; nivolumab; thyroiditis.
©2022 Acta Endocrinologica (Buc).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- De Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Hormone and Metabolic Research. 2019;51(3):145–156. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
