Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments
- PMID: 37152942
- PMCID: PMC10161731
- DOI: 10.3389/fendo.2023.1161521
Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments
Abstract
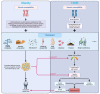
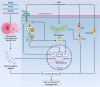
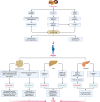
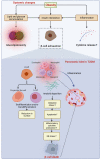
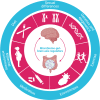
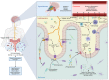
The prevalence of obesity and diabetes mellitus (DM) has been consistently increasing worldwide. Sharing powerful genetic and environmental features in their pathogenesis, obesity amplifies the impact of genetic susceptibility and environmental factors on DM. The ectopic expansion of adipose tissue and excessive accumulation of certain nutrients and metabolites sabotage the metabolic balance via insulin resistance, dysfunctional autophagy, and microbiome-gut-brain axis, further exacerbating the dysregulation of immunometabolism through low-grade systemic inflammation, leading to an accelerated loss of functional β-cells and gradual elevation of blood glucose. Given these intricate connections, most available treatments of obesity and type 2 DM (T2DM) have a mutual effect on each other. For example, anti-obesity drugs can be anti-diabetic to some extent, and some anti-diabetic medicines, in contrast, have been shown to increase body weight, such as insulin. Meanwhile, surgical procedures, especially bariatric surgery, are more effective for both obesity and T2DM. Besides guaranteeing the availability and accessibility of all the available diagnostic and therapeutic tools, more clinical and experimental investigations on the pathogenesis of these two diseases are warranted to improve the efficacy and safety of the available and newly developed treatments.
Keywords: bariatric surgery; diabetes mellitus; islet function; microenvironment; obesity; pathogenesis; β-cell failure.
Copyright © 2023 Ruze, Liu, Zou, Song, Chen, Xu, Yin and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Obesity Federation . World obesity atlas 2022. (London: World Obesity Federation; ) (2022). Available at: https://data.worldobesity.org/publications/?cat=15.
-
- International Diabetes Federation (IDF) . IDF Diabetes Atlas, 10th ed. Brussels: International Diabetes Federation (IDF)). (2022). Available at: https://diabetesatlas.org/atlas/tenth-edition/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

