Patient attitudes and preferences about expanded noninvasive prenatal testing
- PMID: 37152999
- PMCID: PMC10161390
- DOI: 10.3389/fgene.2023.976051
Patient attitudes and preferences about expanded noninvasive prenatal testing
Abstract
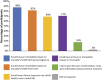
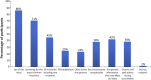
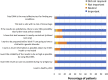
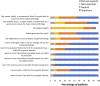
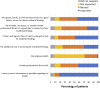
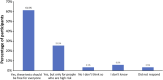
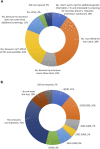
Introduction: Noninvasive prenatal testing (NIPT) using cell-free DNA (cfDNA) is typically carried out to screen for common fetal chromosomal anomalies, with the option to screen for a wider range of chromosomal changes (expanded NIPT) becoming increasingly available. However, little is known about pregnant patients' attitudes and preferences regarding expanded NIPT. Methods: To address this gap, we surveyed general-risk patients having first-tier cfDNA screening at a private prenatal clinic on their expectations for expanded NIPT. Patients were asked questions regarding their current pregnancy and previous pregnancy history, their opinions on fetal DNA screenings during pregnancy and incidental findings, information and opinions on financial resources for NIPT, as well as socio-cultural questions to determine patient demographics. Results: Of the 200 survey participants, the majority were educated, self-reported as white, had a higher than average income, and reported no aneuploidy risk factors. When asked what information they would like to receive from cfDNA screening, the vast majority of participants wanted all information available that could have an immediate impact on fetal health (88%) or an immediate impact on infant health from birth (82%). Many participants also wanted information that could have a future impact on the child's health or an immediate or future impact on the pregnant woman's own health. Most participants wanted information about the sex of fetus (86%) and common trisomies (71%), with almost half of participants desiring information about rare autosomal aneuploidies and/or all genetic information that may affect the baby. In addition, participants were found to be comfortable screening for conditions that are well-known, influence care during pregnancy, and are treatable. Finally, while most respondents either had insurance coverage for NIPT or were able to afford NIPT out of pocket, the majority of our participants felt that expanded NIPT should be either free for everyone or for those considered high risk. Discussion: Our findings suggest that with appropriate pre-test counseling, pregnant patients may choose NIPT for an expanding list of conditions.
Keywords: aneuploidy; cell-free DNA; incidental findings; informed consent; noninvasive prenatal testing; patient preference; surveys and questionnaires.
Copyright © 2023 Dubois, Winters, Rodrigue and Gekas.
Conflict of interest statement
Author PW is an employee of and owns equity in Illumina, Inc. This study received funding from Illumina, Inc. The funder had the following involvement with the study: study design, data analysis, and preparation of the manuscript. Study funding was also used to support a research associate position to implement the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Audibert F., De Bie I., Johnson J. A., Okun N., Wilson R. D., Armour C., et al. (2017). No. 348-Joint SOGC-CCMG guideline: Update on prenatal screening for fetal aneuploidy, fetal anomalies, and adverse pregnancy outcomes. J. Obstet. Gynaecol. Can. 39 (9), 805–817. 10.1016/j.jogc.2017.01.032 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

